
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
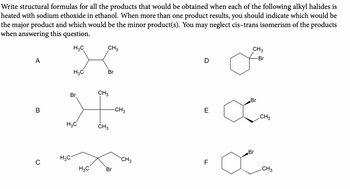
Transcribed Image Text:Write structural formulas for all the products that would be obtained when each of the following alkyl halides is
heated with sodium ethoxide in ethanol. When more than one product results, you should indicate which would be
the major product and which would be the minor product(s). You may neglect cis-trans isomerism of the products
when answering this question.
H3C
CH3
A
H3C
Br
CH 3
-Br
D
Br
CH3
Br
B
-CH3
E
H3C
CH3
0
H3C
H3C
Br
CH3
LL
F
Br
CH3
CH3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 4 images

Knowledge Booster
Similar questions
- The following reaction involves two sequential Heck reactions. Draw structural formu- las for each organopalladium intermediate formed in the sequence and show how the final product is formed. Note from the molecular formula given under each structural formula that this conversion corresponds to a loss of H and I from the starting material. Acetonitrile, CH,CN, is the solvent. 1% mol Pd(OAc), 4% mol Ph,P CH,CN C4H171 C4H16arrow_forwardDraw the structure of the major product for each of the below reactions. If a reactions gives a mixture of products, clearly mention that. Briefly Describe the minor products that result and why. OMe 1) NaNH2 (excess) 2) H₂O HgSO4 in dilute H2SO4 HBrarrow_forwardМЕСНANISMS 1,2-epoxy-1-methylcyclopentane undergoes both acid-catalyzed and base-catalyzed opening of the epoxide ring to form two different products. > Using ethanol as the solvent and an appropriate acid, show the steps in the acid catalyzed mechanism, writing structures for all products of the steps. Circle the major product(s). > Using ethanol as the solvent and an appropriate base, show the steps in the base- catalyzed mechanism, writing structures for all products of the steps. Circle the major product(s). 1,2-epoxy-1-methylcyclopentane CH3arrow_forward
- Provide the structure(s) of the expected major organic product of the reaction shown. 1) Disiamylborane 2) H₂O₂, NaOH OI O II ||| O IV OV CH3CH₂C(CH3)2C=CH OH OH xx xo IVarrow_forwardWrite a mechanism that accounts for the formation of ethyl isopropyl ether as one of the products in the following reaction. CI OEt HCI EtOH Write the mechanism for step one of this reaction. Show lone pairs and formal charges. Only the acidic hydrogen should be drawn out with a covalent bond. Write the mechanism for step two of this reaction (where the product of step one reacts with the solvent, ethanol). Show lone pairs and formal charges. Only the acidic hydrogen should be drawn out with a covalent bond. Write the mechanism for the last step of this reaction (formation of ethyl isopropyl ether). Show lone pairs and formal charges. Only the acidic hydrogen should be drawn out with a covalent bond. CI will act as the base in this reaction.arrow_forward1) Complete the following reaction and provide a detailed, step-by-step mechanism for the process. HBr 2) Provide the structure of the major organic product in the reactions below. HI CH3 win H3C. CH3 CH₂CH3 HCI Xero H₂C CH3 HBrarrow_forward
- According to Hammond's postulate, which of the following is correct? The structure of the transition state of an endothermic reaction will be more similar to the structure of the reagents than to that of the products. The structure of the intermediary in an endothermic reaction will be more similar to the structure of the reagents than to that of the products. The transition state structure of an exothermic reaction will be more similar to reagents than to products. All transition states are more similar to products than reagents All transition states are more similar to reagents than products.arrow_forwardWhich of the reaction conditions could afford the following transformation?arrow_forwardA problem often encountered in the oxidation of primary alcohols to acids is that esters are sometimes produced as by-products. For example, oxidation of ethanol yields acetic acid and ethyl acetate: Propose a mechanism to account for the formation of ethyl acetate. Take into account the reversible reaction between aldehydes and alcohols:arrow_forward
- Write a mechanism that accounts for the formation of ethyl isopropyl ether as one of the products in the following reaction. CI OEt HCI EtOHarrow_forwardPlease help me with this I am very confused and I want to study.arrow_forwardBased on the given conditions, predict the organic product(s) of the following reaction. I highly recommend drawing the mechanism of the reaction before answering. Note: 2-propanol is both the nucleophile and the solvent in this reaction. CH3 CH3 人 HO (2-propanol) CI CH3 CI CH3 CI CH3 CH3 CH3 CH3 CH3 CH3 CH3arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning