
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Plzz... Solve d, e, f parts only. Ignore c part.. I want mechanism and step by step answer ASAP
Plzzz solve all 3 parts. d, e, f otherwise I'll downvote
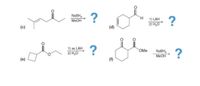
Transcribed Image Text:NABH,
MEOH
?
?
1) LAH
2) H,0
(c)
?
1) xs LAH
NaBH,
MEOH
OMe
2) H,0
?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- order= ??? K= ???arrow_forwardWhat are the Miller indices for the plane drawn in the sketch below?  Select one: a. (∞∞12)(∞∞12) b. (110) c. (00∞)(00∞) d. (010) e. (011)arrow_forwardAutoSave w | homework – Saved to my Mac OFF ... Home Insert Draw Design Layout References Mailings Review View O Tell me R Share O Comments Calibri (Bo. v 11 v A A E vE v E v E E Aa v AaBbCcDdEe AaBbCcD AaBbCcDdE AaBb AaBbCcDdEe Paste BIU V ab x, x A v I v A v No Spacing Normal Heading 1 Heading 2 Title Styles Pane Dictate Sensitivity Please answer the following questions fully and to the best of your ability 1) Please state how you would synthesize the following polymer below and show the mechanism behind the reaction. Note that this also means you need to tell me which molecule you are starting with as well as specifying all conditions required. он OH 2) Will either the polymer or the monomers you used to make it show peaks if IR spectroscopy analysis was done on them, and if so where? How about if UV-vis spectroscopy was performed instead? Page 1 of 1 93 words E English (United States) O Focus 白arrow_forward
- ) 46% Sun 11:04 AM Safari File Edit View History Bookmarks Window Help A session.masteringchemistry.com Ellucian Degree Works... Pearson's MyLab & Mas... ALEKS - Sofia Simmons... MasteringChemistry: H... Consider summer class... Inbox (13) - thesym1@g.. Class Schedule Listing Schedule 111.009 & 111.... View Available Hint(s) Reset Help F2 H2 Mg O2 Oxidizing agent Reducing agent 31410 FEB tv 23 MacBook Pro esc 23 2$ & ....arrow_forwardSafari File Edit View History Bookmarks Window Help () 48% Sun 10:56 AM A session.masteringchemistry.com Consider summer class... Inbox (13) - thesym1@g... Class Schedule Listing Schedule 111.009 & 111.. Ellucian Degree Works... Pearson's MyLab & Mas... ALEKS - Sofia Simmons... MasteringChemistry: H-arrow_forwardGiven that ln(A1) = ln(A0) – kt1 ……….. (i) ln(A2) = ln(A0) – kt2 ……….. (ii) Where A1 = A0 - x1 A2 = A0 - x2 Y = x2 – x1 Prove that x(1)={Ao-[y/(1-e^-k(t2-t1)]} Using SOUND, BALANCED NUCLEAR EQUATION/REACTION AND PRINCIPLE ONLY, explain “How does KI work to help mitigate the effect of exposure to radiation?” http://www.thestar.com/news/article/954546--radiation-fears-spark-run-on-iodide-pills ® ® ® “the SOURCE OF HEAT that resulted in the melt-down at the Fukushima-nuclear-reactor?” [Actual balanced nuclear equations showing heat generated or absence of certain things required for full point]…arrow_forward
- Help 100% 47 T. "ublic Health Ch HSC 258 - Major Projec x MindTap - Cengage Lea X C The Illustration To T =55750828934189288909969212&elSBN=9781305657571&id=D1061392007&nbld=21... * Q Search t Referonces Use the References to access important values if needed for this question. For the following reaction, 50.4 grams of sulfur dioxide are allowed to react with 17.9 grams of water. sulfur dioxide (g) + water (I) sulfurous acid (H2SO3) (g) grams What is the maximum amount of sulfurous acid (H,SO3) that can be formed? What is the FORMULA for the limiting reagent? grams What amount of the excess reagent remains after the reaction is complete? Submit Answerarrow_forwardQ3 we dilute unknown sample of 25ml to 100ml And want to find error propagation associated with it. The unknown sample concentration is :1.2984 ppm Error: .1958 Class A 25 ml pipet error : .03ml Class A 100ml beaker : .08 I find the unknown concentration but need error associated with it during dilution process Any hints?arrow_forwardFor a disc-shaped particle of diameter 2.00mm and length 0.500 mm, calculate the following diameters:a. the equivalent volume sphere diameter;b. the equivalent surface sphere diameter;c. the surface-volume diameter (the diameter of a sphere having the same external surfaceto volume ratio as the particle) d. What is the sphericity of the particle?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY