
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:r
5 2 ✓ C
Home Insert Draw Design
AutoSave OFF
Paste
Page 1 of 1
Calibri (Bo... v 12
B I
0 words
V
4
ab X₂
V
Layout
A^ A
x² A
▲Hrxn
V
=
References
Aa ✓
Dv
1
English (United States)
V
Mailings Review View
B-E 1¹E EE
V
V
Tell me
A↓
Document1
3
Aa BbCcDdEe
Normal
AaBbCcDdEe
No Spacing
4
I +
Aa BbCcDc AaBb CcDd E€ Aa Bb
Heading 1
Heading 2
Title
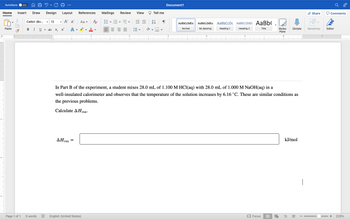
In Part B of the experiment, a student mixes 28.0 mL of 1.100 M HCl(aq) with 28.0 mL of 1.000 M NaOH(aq) in a
well-insulated calorimeter and observes that the temperature of the solution increases by 6.16 ˚C. These are similar conditions as
the previous problems.
Calculate AHxn.
5
↓
Styles Dictate
Pane
6
Focus
kJ/mol
!!!!!
||||
I
Share
Comments
Sensitivity Editor
+ 220%
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 3. Two solutions, 20.00 mL of 2.50 M HCl(aq) and 20.00 mL of KOH(aq) of 2.50 M KOH(aq) both initially at 21.0 °C, are added to a Styrofoam-cup calorimeter and allowed to react. The temperature rises to 37.8 °C. Determine the heat of reaction, AHxn, in J per moles of HCI. Is the reaction endothermic or exothermic? (Hint: dsolm = 1.021 and Csol'n = 4.122; this problem involves same calculations as done for the experiment). T₁ = 21.0°C mL Chemistarrow_forwardAs a routine safety procedure, acids and bases should be stored separately in a laboratory. Concentrated hydrochloric acid is 12.0 M and the molarity of 25% ammonium hydroxide is 13.4 M. Both are sold in 2.50 L bottles. If there were an accident and the two bottles broke and the acid and base mixed, how much heat would evolve? State your answer as a positive value. NH4OH(aq) + HCI(aq)→NH4CI(aq) + H2O(1) AH° = -50.49 kJ Heat = ! kJ eTextbook and Media Hint Attempts: 2 of 15 used Submit Answer Save for Later Last saved 1 day ago. Saved work will be auto-submitted on the due date. Auto- submission can take up to 10 minutes. átv OCT 26arrow_forwardAs a routine safety procedure, acids and bases should be stored separately in a laboratory. Concentrated hydrochloric acid is 12.0 M and the molarity of 25% ammonium hydroxide is 13.4 M. Both are sold in 2.50 L bottles. If there were an accident and the two bottles broke and the acid and base mixed, how much heat would evolve? State your answer as a positive value.NH4OH(aq) + HCl(aq) → NH4Cl(aq) + H2O(l) ∆H° = -50.49 kJarrow_forward
- A 100.0 mL sample of 0.250 M NaOH is mixed with a 150.0 mL sample of 0.280 M HNO3 in a coffee cup calorimeter. Both solutions were initially at 35.00°C and after mixing, the temperature of the resulting solution was recorded as 37.00°C. Determine the enthalpy of neutralization (ΔH°neutralization rxn) (in units of kJ/mol) for the neutralization reaction between aqueous NaOH and HNO3. The specific heat capacity of water = 4.184 J/g°C and density of water = 1.00 g/mL Assume 1) that no heat is lost to the calorimeter or the surroundings, and 2) that the density and the heat capacity of the resulting solution are the same as water. Hint: Heat of neutralization is the enthalpy change per mol of water formed in a neutralization reaction. Write the balanced equation, calculate the limiting reactant and the mol of water formed in the reaction. Enter the numerical value clearly indicating whether it is endothermic or exothermic.arrow_forward50.0 mL of 2.03 M NaOH(aq) is mixed with 50.0 mL of 2.20 M HCl in a Styrofoam calorimeter. Calculate the moles of NaOH(s) added to the calorimeter. The molar mass of NaOH is 40.00 g/mol, H2O is 18.016 g/mol, an HCl is 36.458 g/mol. Report your value to the correct number of significant figures.arrow_forwardA quantity of 2.00 ×102 mL of 0.715 M HCl is mixed with 2.00 × 102 mL of 0.358 M Ba(OH)2 in a constant-pressure calorimeter of negligible heat capacity. The initial temperature of the HCl and Ba(OH)2 solutions is the same at 21.39°C. For the process below, the heat of neutralization is −56.2 kJ/mol. What is the final temperature of the mixed solutions? H+(aq) + OH−(aq) →H2O(l)arrow_forward
- A student dissolves 14.9 g of sodium hydroxide (NaOH)in 300. g of water in a well-insulated open cup. He then observes the temperature of the water rise from 21.0 °C to 33.2 °C over the course of 5.4 minutes. Use this data, and any information you need from the ALEKS Data resource, to answer the questions below about this reaction: NaOH(s) → Na"(aq) + OH (aq) You can make any reasonable assumptions about the physical properties of the solution, Be sure answers you calculate using measured data are rounded to 3 significant digits. Note for advanced students: it's possible the student did not do the experiment carefully, and the values you calculate may not be the same as the known and published values for this reaction. O exothermic Is this reaction exothermic, endothermic, or neither? O endothermic O neither If you said the reaction was exothermic or endothermic, calculate the amount of heat that was released or absorbed by the reaction in this casearrow_forwardWhen a 4.25-g sample of solid ammonium nitrate dissolves in 60.0 g of water in a coffee-cup calorimeter, the temperature drops from 22.0 °C to 16.9 °C. Part A Calculate AH (in kJ/mol NH,NO3) for the solution process Thermometer NH,NO3 (s) - NH (aq) + NO, (ag) .Glass stirrer Assume that the specific heat of the solution is the same as that of pure water. Cork stopper Express your answer to two significant figures and include the appropriate units. Two Styrofoam cups nested together X•10" Reaction mixture in solution Value Unitsarrow_forward34, Considering the limiting reactant, how much heat is produced at constant pressure if 0.243 g of magnesium reacts with 100.0 mL of 0.300 M HCl according to the reaction below? Mg(s) + 2HCl(aq) => MgCl2(aq) + H2(g); ΔHo = –462.5 kJ Group of answer choices A, 23.1 kJ B, 13.9 kJ C, 4.63 kJ D, 9.24 kJ E 6.94 kJarrow_forward
- THERMODYNAMICS: The combustion of 1.00 mol of glucose (CGH1206) liberates 2820 kJ of heat. If 1.38 g of glucose is burned in a calorimeter containing 801 g of water, and the temperature of the assembly increases from 20.10°C to 24.21°C, what is the heat capacity of the calorimeter in kJ/°C? MW of glucose is 180 g/mol. (Round off the final answer to TWO decimal places). Round your answer to 2 decimal places. Add your answerarrow_forwardCalculate the heat of neutralization (kJ/mole) for the reaction of 50.0 mL of 0.177 M potassium hydroxide with 100.0 mL 0.841 M sulfuric acid resulting in a temperature increase of 8.22 oC. Assume the solutions have a density of water and specific heat capacity of water, 4.184 J/(g⋅oC). The calorimeter is a perfect system with no heat loss. 2KOH + H2SO4 ⟶ K2SO4 + 2H2Oarrow_forward150 mL of a 0.728 M HCl aqueous solution is mixed with 150 mL of 0.364 M Ba(OH)2 aqueous solution in a coffee-cup calorimeter. Both the solutions have an initial temperature of 26.5°C. Calculate the final temperature of the resulting solution, given the following information: H+(aq) + OH- (aq) → H2O(ℓ) ΔHrxn = -56.2 kJ/mol Assume that volumes can be added, that the density of the solution is the same as that of water (1.00 g/mL), and the specific heat of the solution is the same as that for pure water, 4.184 J/(gK).arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY