
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
This question was rejected, but as you can see it's not wort credit the point total says it's out of zero
Transcribed Image Text:WA Practice 4 - CHEM 1201 X
co Course: 2020 Spring CHEM
b My Questions | bartleby
A https://www.webassign.net/web/Student/Assignment-Responses/randomize?pos=14&dep=22671454&tags=autosave#question361117_14
...
15.
0/0 POINTS
PREVIOUS ANSWERS
21/100 Submissions Used
MY NOTES
ASK YOUR TEACHER
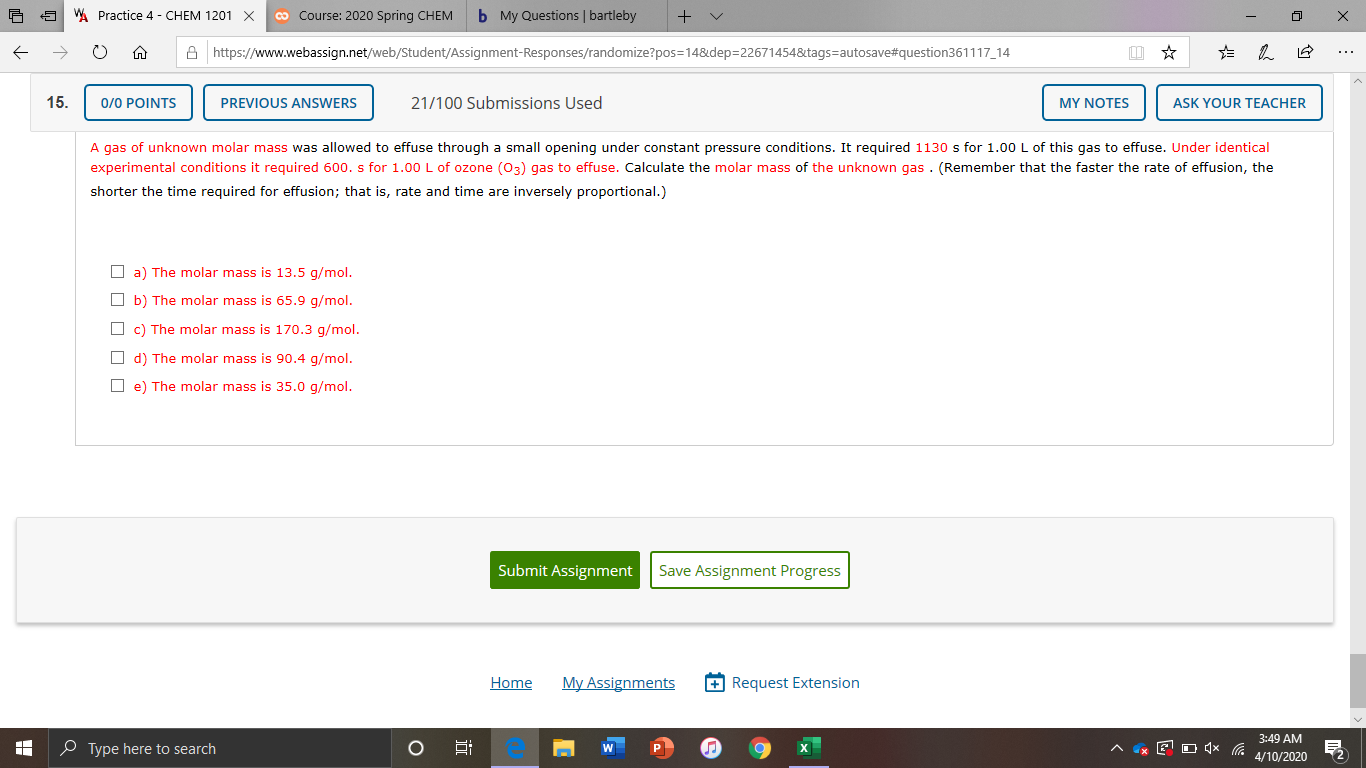
A gas of unknown molar mass was allowed to effuse through a small opening under constant pressure conditions. It required 1130 s for 1.00 L of this gas to effuse. Under identical
experimental conditions it required 600. s for 1.00 L of ozone (03) gas to effuse. Calculate the molar mass of the unknown gas . (Remember that the faster the rate of effusion, the
shorter the time required for effusion; that is, rate and time are inversely proportional.)
O a) The molar mass is 13.5 g/mol.
O b) The molar mass is 65.9 g/mol.
O c) The molar mass is 170.3 g/mol.
O d) The molar mass is 90.4 g/mol.
O e) The molar mass is 35.0 g/mol.
Submit Assignment
Save Assignment Progress
Home
My Assignments
+ Request Extension
3:49 AM
O Type here to search
4/10/2020
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Enter the units of r when the amount is measured in mol L-1 and time in min (use the backslash / but be sure to use () when more than one unit is is the denominator and a space or * to mean the product, for example: L/(mol s) or L/(mol*s) or L/(s mol) or L/(s*mol) )arrow_forwardPlease answer this question below and make sure that all work shown on paper and is in a correct ''Dimensional Analysis Format''. Make sure that it is answered correctly. (dont answer if its not in a dimensional analysis format, and dont just give an answer, all work shown) Question: FInd the Molar Mass for Phosphorus Pentachloride (PCl5)arrow_forwardPlease calculate and mke the numbers ledgibe... I GOTTHIIS ANWER YOU PROVIDED WRONG AND 2 SIG FIGSARE NEEDED ALSOarrow_forward
- The solution calls for an exponent. How would I write that?arrow_forwardWhy does the x go from:-0.9968x = -0.0025To:x= 0.0025x/0.9968And not:x= 0.9968/0.0025Wouldn't the negatives simply be ignored and the x would remain with:0.9968x and then you divide then 0.0025 without the coefficient x to get the value of x by cancelling out the 0.9968?arrow_forwardChemistry How to balance centrifuge with odd number of vials with no balancing vials. Given 24 slots, with 7, 9, 11, 13, 15, 17, 19 vials.arrow_forward
- CUTICLE OIL O Microsoft W final E.A 1. G fahrenheit x 9 Learning M X O Schoology x O ch 9 key A mukilteo.schoology.com/common-assessment-delivery/start/4592487484?action on.. Bb Molar Mie O Periodic x 3-6, 6-5, & 6-6 Test 6 of 10 © 29 POSSIBLE POINTS: 2 What is the mass of a 5.521 mole sample of MgCl2? O 17.26 g O 100.7 g O 525.7 g O 256.1 g 4. 6 8 9 10 Lenovo DI 23 $ 7 4 t r e ーの づarrow_forwardDetermine the molar mass of Cu(NO,),. Provide an answer to two decimal places.arrow_forwardA particle is immersed in a gas. What would happen with the gas pressure the particleexperiences if the density of the gas was halved?. Explain.arrow_forward
- I have calculated this problem various times, but every time I input the answer into the assignment it says it's wrong, I can't find the issue. What could it be?arrow_forward2. A donut contains 37.8 grams of sucrose (C12H22O11). How many moles of sugar are in the donunt? Example attached and significant figure rules. Be SURE to apply the significant figure rules to your final answer. Also write how many significant figures each answer has.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY