
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
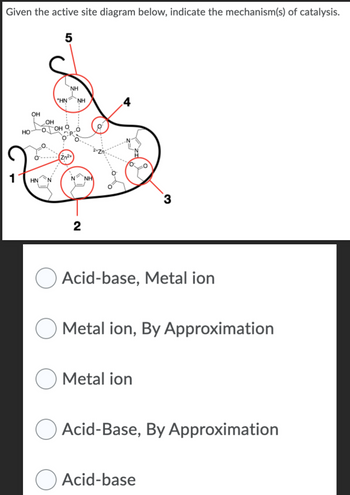
Transcribed Image Text:Given the active site diagram below, indicate the mechanism(s) of catalysis.
5
HO
OH
HN
OH
ΝΗ
*HN ΝΗ
Zn²+
Acid-base, Metal ion
3
Metal ion, By Approximation
Metal ion
Acid-Base, By Approximation
Acid-base
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Discuss in a short paragraph (3–5 sentences for each mutation) whether or not the enzyme is likely to completely lose catalytic activity if the following mutations are carried out. You may assume that steric effects do not distort the active site so much that catalysis cannot take place; focus your analysis on discussing the chemistry the amino acid side chains are able to perform and the properties that enable them to do so. Mutation 1: H to E Mutation 2: H to N Mutation 3: S to D Mutation 4: S to Carrow_forwardIf you can clearly visualize the chymotrypsin mechanism of action, you should be able to picture the structure of the transition state right after the enzyme attacks the first substrate. Think hard about what we have covered, and visualize that transition state accurately:arrow_forwardIn a typical enzyme-catalyzed reaction, what are the relative concentrations of reactants and products as compared to the enzyme concentration?arrow_forward
- Inhibitors are compounds capable of blocking the catalytic process. Outline with the use of graphs and equations in illustrating the different modes of action of enzyme inhibitors.arrow_forwardCan you explain the following based on its mode of enzyme kinetics: 1) Synthesis/degradation of enzymes 2) Allosteric regulation 3) Covalent modificationarrow_forwardConsider the following free energy diagram for an uncatalyzed and enzyme-catalyzed reaction. Select all the statements that are true. Without enzyme With enzyme A+B Time AB Oa. The reaction is now spontaneous due to the addition of enzyme b. The rate of the enzyme catalyzed reaction is faster than the uncatalyzed reaction O C. The reaction is exergonic O d. The change in free energy for the reaction is greater in the catalyzed reaction, compared to the uncatalyzed reaction e. The enzyme stabilizes the transition state for the reaction Released Energy pesarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON