
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Letter K pleaseeee
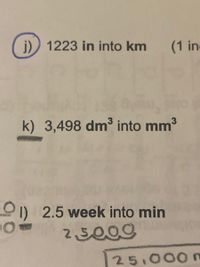
Transcribed Image Text:j)) 1223 in into km
(1 in
k) 3,498 dm³ into mm3
O) 2.5 week into min
25009
25,000
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please show all the steps in solvingarrow_forwardConyugated acid Conyugated bese Jamula ha Formula ha A NH 5.61010 CH,NH HCO 2.2 10-8 Complete the table below. B Sure cach OA Your cinowrentries has the Correct numbCr oA Signiticant digits. You may assime the tamparature is 25°C.arrow_forwardVilin the felowina Chart wtn the tonyerature as the udume Charges Hepl:Find the qudient if ne whne diluided by the Seemifercature in mefustrow.Use this figure as H. Sep 2 Use me tanula v/T=h to sohe for the ather tempratures. K hill rewidn Consiant oa a saNse Temperatine/lehuny 120 aso ons 125.arrow_forward
- envellum.ecollege.com/course.html?courseld=15432274&HepID=2b3e48e6520860bfd5591538a4a5a27b#10001 Search... Ael. AOL Video- Serving the best vi.. nline Sh... TripAdvisor 33 of 40 I Review | Constants | Periodic Table Learning Goal: To become familiar with the concept and calculations of specific heat. Part A Specific heat (which can be represented as SH, Cs, sp. ht., or a number of other possibilities) is defined as the amount of energy needed to raise the temperature of 1 g of a substance by 1 °C. For example, 0.0920 cal is enough energy to raise 1 g of copper from 21.0 °C to 22.0 °C. Therefore, the specific heat of copper is 0.0920 cal/(g - °C). How much heat energy is required to raise the temperature of 0.360 kg of copper from 23.0 °C to 60.0 °C? The specific heat of copper is 0.0920 cal/(g- °C). Express your answer with the appropriate units. > View Available Hint(s) Using SH for specific heat, the formula for calculating specific heat is HA Value Units heat heat = SH = massxAT P…arrow_forwardK Haylee Scott - cp Zoom meeting lin com/web/viewer.html?state%=D%7B'ids %3A%5B"1zOflgo1GoQFrdviJ6PWx8L3h2j4uav5n"%5D%2C"action"%3A'open C Clever | Portal A Classroom https://sso.theleam. M Oro Grande Elemen. W Yearbook Aven ue Period 5 Scien. Haylee Scott - cprr061.pdf Building Vocabulary From the list below, choose the term that best completes each sentence. matter physical change endothermic reaction chemical change chemistry precipitate exothermic reaction 7. Any change that alters a substance without changing it into another substance is a(n) 8. is anything that has mass and takes up space. 9. A reaction that releases energy in the form of heat is called a(n) 10. A(n) absorbed. 11. A chemical reaction is also referred to as a(n) is a reaction in which energy is 12. A(n) a chemical reaction. is a solid formed from a solution during 13. is the study of the properties of matter and how matter changes. OPearson Education, Inc., publishing as Pearson Prentice Hall. All rights reserved…arrow_forwardPlease calculate and mke the numbers ledgibe... I GOTTHIIS ANWER YOU PROVIDED WRONG AND 2 SIG FIGSARE NEEDED ALSOarrow_forward
- A ALEKS - Alec Nema - Learn G Complete the table below, which x C Complete The Table Below, Whici x + > C O A www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-lgNslkr7j8P3jH-IvUrTNdLZh5A8CnG03PBGuXr8iCPa7ZMmym9dT1il1Gkroy7AtUHLxV9FND2YCHj_luf5tjUsCtVPrkHH_G.. Apps Sprouts Academy. O Online Tutoring C 400 Request Heade. Q Weather & Soil CH. O THERMOCHEMISTRY Solving combustion thermochemistry problems Alec v On a 10 day wilderness expedition you'll need to heat 3.0 kg of water to the boiling point each day. The air temperature will average 25 °C. You have available canisters of compressed propane (C,Hg) fuel, which you'll burn to heat the water. Each canister has 50. g of propane in it. What is the minimum number of fuel canisters you must bring? The standard heat of formation of propane at 25 °C is -103.85 kJ/mol. You'll probably find other helpful data in the ALEKS Data resource. 圖 dlo canisters: Exliyanation Check O 2020 McGraw-Hill Education. All Rights Reserved. Terms of Use| Privacy |…arrow_forwardarrow_forwardits incorrectarrow_forwardNeed help with homeworkarrow_forward45. | You find that if you hang a 1.25 kg weight from a vertical spring, it stretches 3.75 cm. (a) What is the force constant of this spring in N/m? (b) How much mass should you hang from the spring so it will stretch by 8.13 cm from its original, unstretched length?arrow_forwardO Course Home b Chemistry Question | bartleby G indivuials with austium good to -> A openvellum.ecollege.com/course.html?courseld=16561285&OpenVellumHMAC=446c877376a9bc7ac878d8ad119f4717#10001 Q * E Apps O Maps E Connect - To Do As... O OCCC Moodle P chem work b help Gmail YouTube Balance Chemical E. II Review | Constants | Periodic Table A carbon atom is chiral if it is bonded to four different groups. For example, CHCIBII is chiral, but CCl,BrI is achiral because some of the bonded groups are the same. If a chiral carbon atom is present, then that molecule has a non-superimposable mirror image called an enantiomer. (Figure 1) Part A Identify all the chiral atoms in the below structure by right-clicking* a chiral atom to bring up a menu that includes "Atom Properties." Click on Atom Properties then click the checked box next to the Map field to clear the checkmark. Then enter "1" in the Map *Mac users: Use an equivalent for right- field box to label that chiral carbon atom. clicking.…arrow_forwardarrow_back_iosSEE MORE QUESTIONSarrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY