
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question

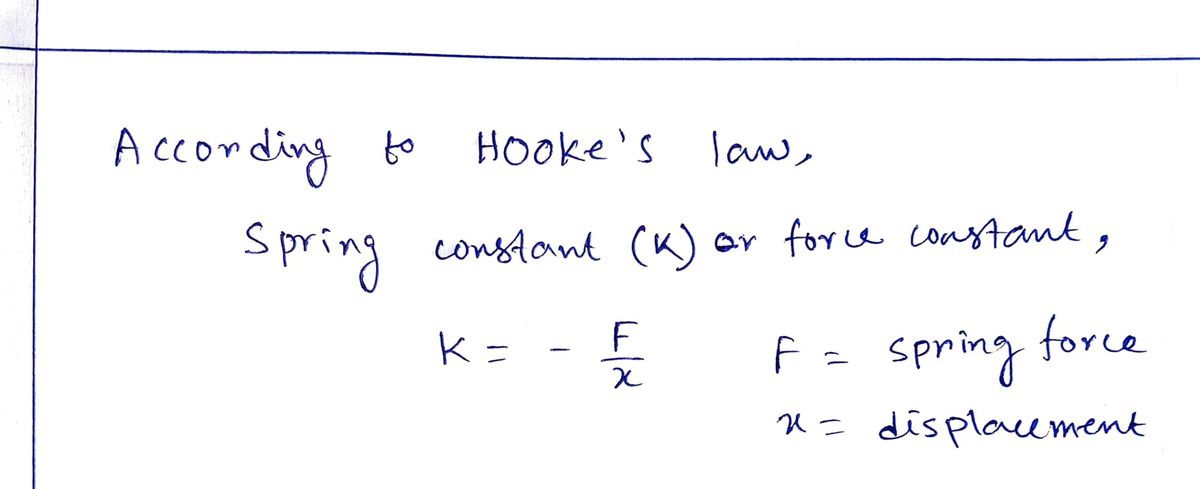
Transcribed Image Text:45. | You find that if you hang a 1.25 kg weight from a vertical spring,
it stretches 3.75 cm. (a) What is the force constant of this spring in
N/m? (b) How much mass should you hang from the spring so it
will stretch by 8.13 cm from its original, unstretched length?
Expert Solution
arrow_forward
Step 1
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- While excavating ancient remains, an archaeologist discovers a box of minted coins. The nineteen coins have identical markings and composition. The archaeologist performs some measurements and records the following data in her lab notebook: total mass of coins = 1.23 lbs melting point of material = 1981 °F coins dropped into graduated cylinder, water rises from 3.50 fl oz. to 5.62 fl oz. Later, the archaeologist compares her measurements to the data table shown below. Is it possible the coins are made of one of the metals listed in the table? If so, which one? Consider the information to the right of the table. Metal metal alloy #1 metal alloy #2 metal alloy #3 metal alloy #4 Density (g/cm3) 10.58.910.58.9 Melting Temp (°C) 10839629621083 (1 fluid oz. = 29.57 mL) (454 g = 1 lb)arrow_forwardAt 36.0 °C the density of water is 0.9936870 g/cm3.At 37.0 °C the density of water is 0.9933390 g/cm3.Use linear interpolation to calculate the density (D) of water at 36.2 °C, to the nearest 10-7g/cm3.D =arrow_forward5) How many minutes is required for sunlight to travel from the Sun to Mars? (Assume the Sun is 1.14 x 10^8 km from Mars and that sunlight travels at 2.99 x 10^5 km/s.)(a) 0.00437 minutes (b) 0.157 minutes(c) 6.35 minutes (d) 381 minutes(e) 22,900 minutes Step by step use the conversion factors with starting point and put lines in between the numbers and cancel the units 6) Platinum is one of the most dense elements (d = 21.5 g/cm^3). What is the volume of a 10.0 g sample of the metal?(a) 0.465 cm^3(b) 2.15 cm^3(c) 21.5 cm^3(d) 215 cm^3(e) 465 cm^3 Step by step, use the conversion factors with starting point and put lines in between the numbers and cancel the units 7) A rare metal alloy is a superconductor at 77 K. What is the temperature in °C ?(a) -350°C (b) -196°C(c) -77°C (d) 196°C(e) 350°C Step by step use the conversion factors with starting point and put lines in between the numbers and cancel the units 8) The average distance from the Earth to the Sun is 1.50 x 10^8km.…arrow_forward
- Question 2. Alldredge studies the properties of marine snow in Ref. [2]. The figure below is adapted from this work, and plots the mass of different particles of fecal marine snow as a function of their volume on a log-log scale. In[Particle Mass M (ug) / (1 µg)] 5.0 4.5 4.0 3.5 3.0 2.5 2.0 1.5 1.0 ● 0 Fecal snow Line of best fit 2 3 4 5 In[Particle Volume V (mm³) / (1 mm³)] 6arrow_forwardcalculate the volume occupied by 25.0 g of lead if the density of lead is 11.4g/cm^3 at 25 degrees celsius.arrow_forwardA 270.3 mL sample of carbon dioxide was heated to 303 K. If the volume of the carbon dioxide sample at 303 K is 519.6 mL, what was its temperature at 270.3 mL? T = Karrow_forward
- A 540.3 mL sample of carbon dioxide was heated to 307 K. If the volume of the carbon dioxide sample at 307 K is 789.6 mL, what was its temperature at 540.3 mL?arrow_forwardA 2.02 g lead weight, initially at 10.6 °C, is submerged in 8.21 g of water at 52.2 °C in an insulated container. Express the temperature in Celsius to three significant figures. View Available Hint(s) ΤΟ ΑΣΦ 0 ?arrow_forwardA student finds that 24.96 g of water at 24.9 C(density=0.9971 g/cm3) is required to completly fill an empty flask. The water is removed and completely dried; granular solid copper weighing 51.24g is then added to the flask. With the copper present in the flask, it was determined that 19.24 g of water was required to fill the remaining space in the flask completly 1.) volume of the empty flask 2.) volume of the copper 3.) density of the copperarrow_forward
- 5) How many minutes is required for sunlight to travel from the Sun to Mars? (Assume the Sun is 1.14 x 10^8 km from Mars and that sunlight travels at 2.99 x 10^5 km/s.)(a) 0.00437 minutes (b) 0.157 minutes(c) 6.35 minutes (d) 381 minutes(e) 22,900 minutes Step by step use the conversion factors with starting point and put lines in between the numbers and cancel the units 6) Platinum is one of the most dense elements (d = 21.5 g/cm^3). What is the volume of a 10.0 g sample of the metal?(a) 0.465 cm^3(b) 2.15 cm^3(c) 21.5 cm^3(d) 215 cm^3(e) 465 cm^3 Step by step, use the conversion factors with starting point and put lines in between the numbers and cancel the units 7) A rare metal alloy is a superconductor at 77 K. What is the temperature in °C ?(a) -350°C (b) -196°C(c) -77°C (d) 196°C(e) 350°C Step by step use the conversion factors with starting point and put lines in between the numbers and cancel the units 8) The average distance from the Earth to the Sun is 1.50 x 10^8km.…arrow_forward(a) Multiply 2.334 cm and 0.320 cm.(b) Divide 55.8752 m by 56.53 s.arrow_forwardHow many grams of water can be cooled from 39 °C to 19 °C by the evaporation of 44 g of water? (The heat of vaporization of water in this temperature range is 2.4 kJ/g. The specific heat of water is 4.18 J/g.K.) Express your answer using two significant figures. m = 196) ΑΣΦ ? garrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY