
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
I'm just asking about 2 and 3 but I assume you need the web to answer them.
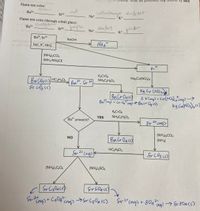
Transcribed Image Text:Write the proce
step
by each
Flame test color:
gren
ved
yellowforuge
blue violet
K+
Ba2+
Sr2+
Na+
Flame test color (through cobalt glass):
Ba?+ Llu/gvein
Sr²+ _ }umple
color lers
Sr2+
Na+
K+
Ba, Sr2+
NaOH
iglo
10
Na", K", NH.
NHA"
+4
h ot onab be frn A
a od banno
alb di
(NH4)2CO3
(NH3/NH,CI)
K2CrO4
NH,C2H02
HC2H3O2
Na;Co(NO2)6
Ba2, Sr 24
Kg Co (NO26
3 Fiaup + COLNO)cag>->
BaCrO4s)
2+
Ba
KCrO4
YES
NH,C2H3O2
Ba2+
present?
2+
(NH)2CO3
NO
Bacr O4cs)
(NH3)
HC,H;O2
SrZt
Cag)
Sr CO3 cs)
(NH4)2GO4
(NH4)2SO4
Cz04°
2-
2+
cag>
2-
cay)
caq> + 804
(ag) Sr804(s)

Transcribed Image Text:184
Qualitative Analysis: Group III
Tolon
1259 0m
3. A student treated the decantate from first step of the procedure above with Na,Co(NO2)6 and observed that a precinitate
formed. The student reported K+ to be present in the unknown. Do you agree with this student's conclusion? Why on
why not?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- I put the question in 2 pictures!arrow_forwardWhat is the wavelength, in nanometers, of light with an energy content of 2.89 x 102 kJ mol-1? Hint: The unit mol1 should be interpreted as "per mole of photons". Enter your answer with three significant figures. Enter scientific notation as 1.23E4. Énter only numbers in your answer. (Do dot include units.) Number nm Ouesticarrow_forwardTwo particles with charges ?1q1 and ?2q2 are separated by distance ?.d. Arrange these scenarios according to the magnitude of the electrostatic (coulombic) potential energy. Ignore sign.arrow_forward
- x MyLab and Mastering J Course Home MyLab and Mastering envellum.ecollege.com/course.html?courseld316985674&OpenVellumHMAC=Db6918a05d7d0ddc49a113faa2dba85b0# 10001 I Review | Constants | Periodic Table You may want to reference (Pages 420 - 425) Section 10.4 while completing this problem. Part A A chemical reaction occurring in a cylinder equipped with a moveable piston produces 0.641 mol of a gaseous product. If the cylinder contained 0.240 mol of gas before the reaction and had an initial volume of 2.19 L, what was its volume after the reaction? (Assume that pressure and temperature are constant and that the initial amount of gas completely reacts.) Express the volume to thee significant figures and include the appropriate units. Units Submit Previous Answers Request Answer X Incorrect; Try Again; 4 attempts remaining Provide Feedback Next2 P Pearson Copyright © 2021 Pearson Education Inc. All rights reserved. | Terms of Use | Privacy PolicyI Permissions Contact Us| search 78°F…arrow_forwardBe sure to answer all parts. What is the energy (in J) of one photon of 833 nm wavelength light? Enter your answer in scientific notation. _____x10^Jarrow_forwardPls help me solve the following questiojn, explain it, thank yiou!!arrow_forward
- 1:25 A O * N 5GUC I 67%, Show What You Know: Learning... KO> O 8 de 8 A new band sensation is playing a concert and recording it for a live album to be released this summer. The band asks the sound mixer if he can autotune the singer who has a habit of singing slightly lower than the note. What would the mixer need to do to the sound wave? How would it affect the characteristics of the wave? В I |Revisión 田 !!!arrow_forwardChrome File Edit View History Bookmarks Profiles Tab Window Help 31% Fri 10:35 PM sciencenotes.org 101 Chem101 Identify the region o x b My Questions | bart X CHEM 111-Ch 3 Flas X + 1 18 Update : IA VIIIA app.101edu.co 1A 8A Periodic Table of the Elements -1, +1 Apps M Gmail" YouTube Translate P MyLabsPlus | Pear... TE MYTCC Blcackboard Reading List H Не >> 2 13 IIIA ЗА 14 15 16 17 IIA IVA VA VIA VIIA Hydrogen 1.008 Atomic Number Helium 4.003 Valence 2A 4A 6A 5,-3,-3 8 5A 7A 4 5 +4,+3,+2,+1 -110 +1 +2 +3 3 7 Symbol 4,-3 -2,-1 Question 18 of 50 Submit Li Ве F Ne Lithium 6.941 Beryllium 9.012 Carbon Oxygen 15.999 Boron Nitrogen 14.007 Fluorine 18.998 Neon 20.180 Name Atomic Mass +4,-4 15 *5,*3,-3 16*6,+4,+2,-217*7.+5,+3,1 18 +1 +2 13 +3 11 12 14 Na Mg AI Si ci| Ar 3 IIIB ЗВ 4 IVB 4B 6 VIB 6B 7 VIIB 7B 8 10 Identify the region of the electromagnetic spectrum where the wavelength VB 5B VI 8 11 IB 1B 12 IB 2B Magnesium 24.305 Silicon 28.086 Sulfur 32.066 Chlorine 35.453 Argon 39.948…arrow_forward337.1 nm(wavelength of a nitrogen laser) Express your answer using three significant figures.arrow_forward
- measured to have a wavelength of (3.51x10^2) nm. What is the frequency • the correct number of significant figures • written in correct scientific notation Note: Your answer is assumed to be reduced to the highest power possible. Your Answer: -9 8.55 MHz x10 of the light (in Hz)? c = 3.00 x 108 m/s 1 Hz = 1 s 1 Be careful with your units!!! For full credit, your answer must be:arrow_forwardBzbasbbssarrow_forward12. Which of the following has the largest radius? A) Ве B) С C) О D) F E) Nearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY