
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
predict the prduct
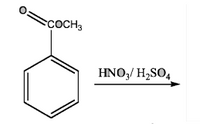
Transcribed Image Text:sCOCH3
HNO,/ H,SO,
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 1) A gas at a pressure of 10.0 Pa exerts a force of A) 55 B) 0.55 C) 5.5 D) 1.8 E) 18 N on an area of 5.5 m2.arrow_forwardCalculate the kinetic energy (in Joules) of 2.000 mole of hydrogen gas at 450.0K.arrow_forward2. Find the final temperature of a 2.00-L gas sample at 20.00C cooled until it occupies a volume of 500mL.arrow_forward
- LoNow.let's buk at Ahe data for the relationship between RIVDE Dse Hhe data trom question 210 Calalate the inverie udume. Fll in the cchant helos witn the data tor the invere f essase Ressuefmmila) 3. 1/3=D0.2 Pressure Immtg) 14 9.4 12.5 18.8 37,7arrow_forwardA 200. ml pyrexflask is filled to thebrim with water witha temperature of 25Celsius. How muchwater (ml) willoverflow when theflask and water isexposed to atemperature of 97Celsius.arrow_forwardA 1.00L sample of cooking has taken from a cylinder was collected. It’s mass, measured at 27 c and 100kPa was 1.768g. Calculate the molar mass of the cooking gas in g/mol.arrow_forward
- 2. Show the Boyle temperature for a van der Waals gas is T Rb Hint: (1- a)-N1+aarrow_forward100 nd X SAsealed balloon is filled with S A sealed balloon is filled with x G celsius to kelvin - Google Sear X + /takeCovalentActivity.do?locator=Dassignment-take VolState e My Account The Common Appli... e elearn 9 YouScience The College Board .. T Login canvas [Review Topica] [References] Use the References to access important values if needed for this question. A sample of fluorine gas has a density of gL at a pressure of 0.580 atm and a temperature of 35 °C Assume ideal behavior. Submit Answer Retry Entire Group 9 more group atternpts lemainingarrow_forwardI know the formula for Kc and that I need to use stoichiometry but I, unsure if I'm doing the math wrong or not setting up the problem correctly. I just want to see how I can set this up to solve myselfarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY