
Human Anatomy & Physiology (11th Edition)
11th Edition
ISBN: 9780134580999
Author: Elaine N. Marieb, Katja N. Hoehn
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Based on the attached figure, calculate the Km value for the enzyme that was tested rounded off to the closest whole number and must include units
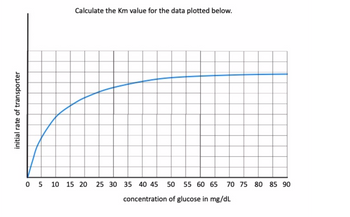
Transcribed Image Text:initial rate of transporter
05
Calculate the Km value for the data plotted below.
10 15 20
25 30 35 40 45 50 55 60 65 70 75 80 85 90
concentration of glucose in mg/dL
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Similar questions
- Hey I am practicing for a molecular biology test and I am having trouble with is practice problem. I am not sure how to set it up to determine the amount of Acid Phosphatase. Can you help? Determine the amount of Acid Phosphatase in micrograms/ gram wheat germ from the following experimental values. The amount of acid phosphatase found in 198 ul of extract was 8 ug. The amount of extraction buffer used was 8 mL and the amount of wheat germ was 0.8 g.arrow_forwardYou perform a Bradford assay. You obtain the absorbance values listed below from the BSA samples; your protein sample yields an absorbance of 1.3; what is the protein concentration of your sample? How did you determine that? BSA (ug/ml) Absorbance @ 595 nm. 25 0.15 50 0.30 75 0.45 100 0.60 150 0.90 200 1.25arrow_forwardIf the enzyme maltase has a Vo of 0.25 mM per minute when [S] = 0.10 mM, and a Vo of 0.40 mM perminute when [S] = 0.40 mM, what is its y-intercept on a Lineweaver-Burk plot?A. 0.10 minutes per mMB. 0.20 minutes per mMC. 0.50 minutes per mMD. 1.0 minutes per mME. 2.0 minutes per mMarrow_forward
- The purpose of the progress curve is to determine if an enzymatic reaction rate remains constant for given concentrations of enzyme and substrate, for a given assay time (20 min). How long did the rate of reaction of WGAP remain constant in your experiments? How do you know? What would cause the reaction rate to not be constant and plateau after some time?arrow_forwardFor the following problem, indicate whether enzyme is repressed (-) or produced (+). Please show all possible outcomes.arrow_forwardTable 1. Kinetic parameters of a chosen model wild-type (WT) enzyme and three catalytically-improved mutants (M1 to M3). Enzyme variants k (8¹) WT MI M2 M3 50 500 1000 50 K₁, (μM) 10 100 100 1 k/K (HM¹¹ s¹) 5 The study above was done on a wildtype enzyme and three genetically-engineered mutants. The purpose was to find a mutation to improve the catalytic efficiency of the enzyme for industrial use. Which is the most accurate statement concerning the data? M2 is the optimal choice when substrate concentrations are saturating. The wildtype enzyme is still preferable to M3 when S<arrow_forwardGive typing answer with explanation and conclusion to all partsarrow_forwardTwo-dimensional gel electrophoresis of proteins in a cell extract provides a qualitative way to compare proteins with respect to intra- cellular abundance. Describe a quantitative approach to the deter- mination of number of molecules of an enzyme per cell.arrow_forwardIn Kjeldahl method, why the protein factors for some other cereal grains (e.g, wheat, oats) differ from that for corn?arrow_forwardWhen you graph your protein assay data in Excel, should you include the absorbance of your unknown BSA sample? Explain why or why notarrow_forwardExplain the Rf value obtained on the basis of the structure of amino acids use. Explain in 1-3 sentence only.arrow_forwardSuppose you have a concentration of 356ng/uL for Sample A. How many ng of protein will that be?arrow_forwardarrow_back_iosarrow_forward_ios
Recommended textbooks for you
 Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON
Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education,
Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education, Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company
Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co.
Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co. Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education
Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education

Human Anatomy & Physiology (11th Edition)
Biology
ISBN:9780134580999
Author:Elaine N. Marieb, Katja N. Hoehn
Publisher:PEARSON

Biology 2e
Biology
ISBN:9781947172517
Author:Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:OpenStax

Anatomy & Physiology
Biology
ISBN:9781259398629
Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa Stouter
Publisher:Mcgraw Hill Education,

Molecular Biology of the Cell (Sixth Edition)
Biology
ISBN:9780815344322
Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter Walter
Publisher:W. W. Norton & Company

Laboratory Manual For Human Anatomy & Physiology
Biology
ISBN:9781260159363
Author:Martin, Terry R., Prentice-craver, Cynthia
Publisher:McGraw-Hill Publishing Co.

Inquiry Into Life (16th Edition)
Biology
ISBN:9781260231700
Author:Sylvia S. Mader, Michael Windelspecht
Publisher:McGraw Hill Education