
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
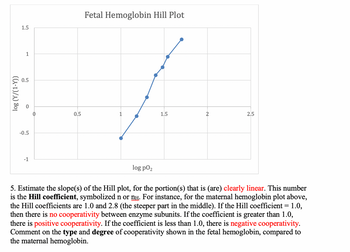
Using the graph data I estimated Slope = 2.8, about the same as the steeper part of the maternal hemoglobin, does this represent a strong positive cooperativity between enzyme subunits?
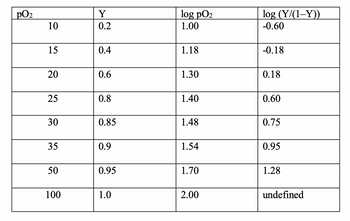
Transcribed Image Text:pO₂
10
15
20
25
30
35
50
100
Y
0.2
0.4
0.6
0.8
0.85
0.9
0.95
1.0
log pO₂
1.00
1.18
1.30
1.40
1.48
1.54
1.70
2.00
log (Y/(1-Y))
-0.60
-0.18
0.18
0.60
0.75
0.95
1.28
undefined
Transcribed Image Text:log (Y/(1-Y))
1.5
1
-0.5
-1
0.5
Fetal Hemoglobin Hill Plot
1
log po₂
1.5
2
2.5
5. Estimate the slope(s) of the Hill plot, for the portion(s) that is (are) clearly linear. This number
is the Hill coefficient, symbolized n or ní. For instance, for the maternal hemoglobin plot above,
the Hill coefficients are 1.0 and 2.8 (the steeper part in the middle). If the Hill coefficient = 1.0,
then there is no cooperativity between enzyme subunits. If the coefficient is greater than 1.0,
there is positive cooperativity. If the coefficient is less than 1.0, there is negative cooperativity.
Comment on the type and degree of cooperativity shown in the fetal hemoglobin, compared to
the maternal hemoglobin.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Similar questions
- If 287.9 umol of enzyme X has a Vmax = 47.8 mmol/sec, what is the value of kcat %3D sec-1? Please report answer with 1 decimal place. Please do not report units. Your Answer: Answer units MacBook Air 888 F5 F4 F3 F2 %23 %24 %24arrow_forward-Inhibitor +Inhibitor [S] (mM) V0&νβσπ;(μmol/sec). νο&νβσπ: &ν βσπ (μmol/sec) 0.0001 33 17 0.0005 71 50 0.001 83 67 0.005 96 91 0.01 98 95 What is the Vmax of this enzyme WITH iinhibitor?arrow_forwardThe table contains data about how different ASFV mutants affect enzyme activity-- for example the D29A mutant, where the D at position 29 was mutated to an A. Select three mutants at random, and for each mutant explain how that mutation could affect intermolecular force interactions (an example: hydrogen bonding could be disrupted by this mutation because…arrow_forward
- 14) Calculate K’eq and ΔG’0 for the following: A 0.1 M solution of glucose 1-phosphate at 25oC is incubated with a catalytic amount of phosphoglucomutase, the glucose 1-phosphate is transformed to glucose 6-phosphate. At equilibrium, the concentrations of the reaction components are Glucose 1-phosphate (4.5 X 10-3 M) and Glucose 6-phosphate (9.6 X 10-2 M).arrow_forwardThe Nutrition Facts label on most food products shows % daily values based on a food energy requirement of 8,360 kJ (2,000 kcal) at normal resting state. a) Assuming that the efficiency of converting food energy to ATP is 50%, calculate the mass of ATP (in kg) that is harvested daily by the human body from 8,360 kJ of food energy. A total of 30.5 kJ of energy is needed to synthesize one mole of ATP under standard conditions. The molar mass of ATP is 505 g/mol. b) For an average 68-kg (150 lbs.) human adult, calculate the % mass of extracted ATP (from your answer in 2a) relative to body weight.arrow_forwardThe transport of aspirin (pKa = 3.5, structure shown here) from the digestive tract to the circulation occurs by nonmediated absorption into cells lining the stomach (where pH = 0.8) and the small intestine (where pH 6.0). Do you expect absorption to be faster in the stomach or in the small intestine?arrow_forward
- Consider a protein with two surface-exposed histidine residues: HisA is a “typical” histidine residue with a pKa = 6.2 HisB is involved in a stabilizing interaction (His-NH+ ..... -O2C-Glu) with a neighboring glutamic acid residue. For HisB, the Gibbs free energy of deprotonation at pH = 7.0 and T = 293K is ΔG'o = +15 kj mol-1. If you had a solution, at pH = 7.0 and T = 293K, containing this protein: a) What fraction of HisA residues are protonated? b) What fraction of HisB residues are protonated? c) What is the pKa of HisB?arrow_forward5.50 1/V, min/umol 5.00 4.50 4.00 y = 0.9474x + 2.6649 y = 0.9997x + 2.032 0.00 1.00 2.00 2.50 3.00 1/[S], uM -1 Looking at the double reciprocal plot for an enzyme in the absence of inhibitor and in the presence of two concentrations of inhibitor, what would be the Vmax for the uninhibited enzyme? (bottom graph) Equation is given. Choose the one best answer. 3.50 3.00 2.50 2.00arrow_forwardThe last residue of the protein (tail) is Tryptophan, and the first residue (head) is labeled with IAEDANS acceptor. Estimate the length of the protein (head to tail) if the efficiency of RET measures at 0.01 Please show step by step and how to get the 34.014 numberarrow_forward
- In a pUC19 digest for 1 ug of pUC19 (DNA conc. 282ng/ul) using 10X Cutsmart buffer, pure water and BamHI enzyme in a total volume of 40 ul, how much of each solution is added to the total volume?arrow_forwardThe KM of a Michaelis-Menten enzyme for a substrate is 1.0 x 104 M. At a substrate concentration of 0.20 M, v = 43 μmol/min. Calculate the rate of reaction when the substrate concentration is tenfold lower, 0.020M. 43 μmol min rate of reaction → Vmax Км substrate concentration →arrow_forwardCalculate θ for a certain protein-ligand pair when the ligand concentration = 1 M and the Kd = 1 X 10-15 M.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON