
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Question
Can you please solve this problem and show all of your work
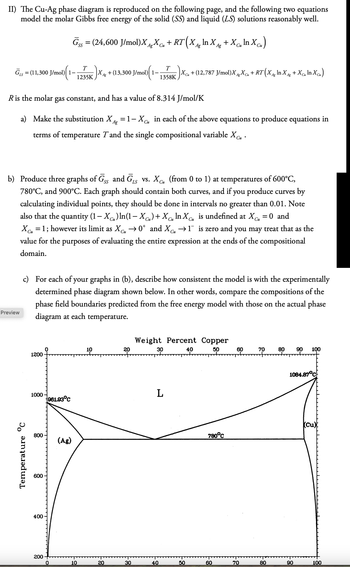
Transcribed Image Text:II) The Cu-Ag phase diagram is reproduced on the following page, and the following two equations
model the molar Gibbs free energy of the solid (SS) and liquid (LS) solutions reasonably well.
Gss = (24,600 J/mol)XX + RT (X^ In X +Xc„ In Xcu)
SS
Cu
Ag
Ag
Cu
1)(1- |Xcμ + (12,787 J/mol)XgXCu + RT (X^g ln X^g +Xcm In Xcu)
T
=
LS
GS (11,300 J/mol) 1-
1235K
Ag
XA + (13,300 J/mol)| 1–
T
1358K
Си
Ag
Ag
R is the molar gas constant, and has a value of 8.314 J/mol/K
a) Make the substitution X = 1-X CM in each of the above equations to produce equations in
Ag
Cu
terms of temperature T and the single compositional variable Xcu.
Си
b) Produce three graphs of GSS and GLS vs. XC (from 0 to 1) at temperatures of 600°C,
Cu
780°C, and 900°C. Each graph should contain both curves, and if you produce curves by
calculating individual points, they should be done in intervals no greater than 0.01. Note
also that the quantity (1-X) In(1-XC)+X In X is undefined at XC = 0 and
Си
Си
Cu
Cu
Си
XC₁ = 1; however its limit as ✗Cu →0* and XC₁ →1¯ is zero and you may treat that as the
value for the purposes of evaluating the entire expression at the ends of the compositional
domain.
Preview
c) For each of your graphs in (b), describe how consistent the model is with the experimentally
determined phase diagram shown below. In other words, compare the compositions of the
phase field boundaries predicted from the free energy model with those on the actual phase
diagram at each temperature.
Temperature °C
10
20
1200
1000
961.93°C
800
(Ag)
600
400
Weight Percent Copper
30
L
200
0
10
20
30
40
50
18-
40
50
60
70
80
90 100
60
1084.87°C
780°C
(Cu)
70
80
90
100
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- At what temperature does the first liquid phase form at 90% Ni? T(°C) 1600 1500 1400 1300 1200 1100 1000, L (liquid) L+α solidus liquidus 80 100 20 40 60 80 wt% Niarrow_forwardI need the solution of this question please please please quickly Thanksarrow_forwardUse the phase diagrams for Cu-Ni and Cu-Ag systems below to answer the question: Based on the mechanism, would you expect a solution containing 80 wt% Cu to be stronger if the remaining 20% of the alloy was Ni or Ag? Briefly explainarrow_forward
- Calculate the weight fraction for phases at point B? what are those phases? Is there any eutectic rection? How many phases are present in this diagram? Cu-Ni system T(°C) TA 1300 -L (liquid): A tie ļine liquidus L +a Тв EB solidus (solid) 1200 TD ID 20 3032 35: 4043 50 Ca wt% Niarrow_forwardFrom the given set of Miller Indices (given below) match each index with corresponding shaded planes according to number. [e.g. 1 match with (ī o 1)] (TOT) (TIT) (To1) (2 Z 2) (002) (3 3 2)arrow_forwardFor the alloy Mg-45 wt% alloy at 500 C, what is the weight percentage of Pb in the alpha phase?arrow_forward
- Use the first image to solve two part questionarrow_forwardA copper-nickel alloy contains 38% by weight of Cu and 62% by weight of Ni and is at 1330 ° C. Using the figure, answer the following questions:a) What is the percentage by weight of copper in the solid and liquid phases at this temperature?b) What percentage by weight of the alloy is liquid and what percentage is solid?arrow_forwardCan you pleae solve this and show all your workarrow_forward
- Problem 3 Given here are the solidus and liquidus tem- peratures for the copper-gold system. Con- struct the phase diagram for this system and label each region. Composition (wt% Au) 0 20 40 60 80 90 95 100 Solidus Liquidus Temperature (°C) Temperature (°C) 1085 1019 972 934 911 928 974 1064 1085 1042 996 946 911 942 984 1064arrow_forwardUse the phase diagrams for Cu-Ni and Cu-Ag systems below to answer the question: Use the diagrams provided to determine the values of the following: i. Solubility of Ni in Cu at 1050 °F. ii. Solubility of Ag in Cu at 700 °F.iii. Solubility of Cu in Ni at 1300 °F. iv. Solubility of Cu in Ag at 400 °F.arrow_forwardQ3 (10 points) Consider a silicon semiconductor at T = 200 K. Determine the electron and hole concentrations for the following cases: (i) (3pts) donor concentration is 10¹5 cm³ and acceptor concentration is 4 × 1015 cm³. (ii) (3pts) donor concentration is 10 15 cm³ and acceptor concentration is zero. (iii) (2pts) donor concentration and acceptor concentration are 4 × 1015 cm-³. (iv) (2pts) donor concentration is 10¹5 cm³ and acceptor concentration is 4 × 1010 cm³.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY