
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
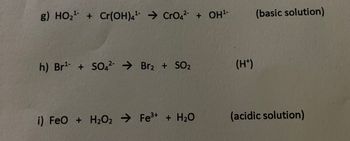
Transcribed Image Text:g) HO₂+Cr(OH)4 CrO42 + OH¹-
h) Br¹ + SO42 Brz + SO2
(H+)
i) FeO + H2O2 Fe³+ + H₂O
(basic solution)
(acidic solution)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- Identify the compound represented by the figurearrow_forwardConsider a HCI solution with a pH of 4.70. a) Calculate the concentration of HCl. b) Identify the two underlying assumptions in part a). c) The original solution is diluted by 1,000 fold. Calculate the pH of the resulting solution. Make sure to indicate if the forementioned assumptions are still valid.arrow_forward9. (1 pt) What is the [H+] and [OH-] for the following strong acid and strong base solutions: A) 0.35 M NaOH B) 1.2 M HCI C) 0.0800 M H2SO4arrow_forward
- Uridine monophosphate (UMP) undergoes a deuterium exchange in deuterated water as shownbelow. Propose a mechanism for this exchange. (arrow_forward3). reactions of the following alkyl iodide. Danny uses potassium tert-butoxide to promote the reaction while Claire uses water and high temperatures. One of their reactions has provided a single elimination isomer while the other has provided a mixture of elimination isomers. Organic chemistry students Danny and Claire are both performing elimination Me H20, heat Danny's Reaction Claire's Reaction H. Me a) Which student's reaction (Danny or Claire) has provided a single elimination isomer? b) Draw all the elimination isomers obtained from both students' reactions. c) Which of the dienes drawn (by you) in question 3b is the most stable? Circle that diene above.arrow_forwardIdentify the structures shown as glycerophospholipids or sphingolipids.arrow_forward
- Fill up the two missing values inside the two circles knowing that the total reaction volume is 2mL. 1) Dilution fraction 2) Water Don't include units in your answer. Membrane Brillant blueR 1g/L Dilution Sample suspension fraction (μL) (μL) 1) Control ? 0 50 2) 1/200 ? 10 50 3) 1/100 0.01 20 50 4) 1/50 ? 40 50 5) 1/20 100 50 Phosphate pH11, 0.2M (ml) 1 1 1 1 1 Water (mL) 0.95 ? ? ?arrow_forwardThe units for the unimolecular reversible rate constant for the forward reaction are ○ M sec-1 sec M-1 sec-1 sec-1arrow_forwardWhich of the following is the cyclic form of an L saccharide? 1) II) CH2OH OH H CH2OH H H H ОН OH H ОН ОН OH H ОН II) IV) H OH ОН CH2OH CH2OH CH2OH H H. H ОН H H H OH ОН ОН OHarrow_forward
- Consider the depeptide below: (look at images) Use first image to answer second image questions.arrow_forwardAn incomplete structure of a porphyrin ring is shown below. The structure is missing three pi bonds and does not show the non-zero formal charges on two atoms. Complete the structure of the porphyrin ring with the missing pi bonds and formal charges. 0 0 +t DC 12⁰ H C N O S F P Cl Br Iarrow_forwardWrite the condensed formula for thisarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON