
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
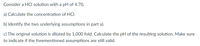
Transcribed Image Text:Consider a HCI solution with a pH of 4.70.
a) Calculate the concentration of HCl.
b) Identify the two underlying assumptions in part a).
c) The original solution is diluted by 1,000 fold. Calculate the pH of the resulting solution. Make sure
to indicate if the forementioned assumptions are still valid.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- B. You will need approximately 1 ml of the 100 µg/ml bromophenol blue (which is your most concentrated standard) to generate your standard curve. Calculate how much bromophenol blue stock solution and how much water you will combine to accomplish this. Make 1 ml of this solution using a 100-1000 μl micropipetter. Use the equation C₁V1=C2V2 μl of 1 mg/ml stock + μl of water = 1 ml of 100 µg/ml bromophenol bluearrow_forwardPrepare 100mL of a 3%m/v NaCl solution. i) What is the total volume of solution wanted? ii) What is the mass of NaCl (solute) needed? iii) What is the volume of water (solvent) needed?arrow_forwardDescribe the preparation of 2.00 L of 0.100 M glycine buffer, pH 9.0, from glycine and 1.00 M NAOH. What mass of glycine is required, and what volume of 1.00 NaOH is required? The appropriate pK, of glycine is 9.6.arrow_forward
- Thimerosal Tincture USP contains 0.1% w/v thimerosal and 50% v/v ethyl alcohol. If the cap is left off of a 15-mL bottle of the tincture, and the ethyl alcohol evaporates leaving a final volume of 9.5 mL, what is the concentration of thimerosal in the evaporated solution expressed as a ratio strength?arrow_forwardWhat is the final volume of NaOH solution prepared from 100.0 mL of 0.500 M NaOH if you wanted the final concentration to be 0.150 M?arrow_forwardCalculate the appropriate volume (in µL) of 9X loading buffer that should be added to 46.0 µL of a sample prior to loading the sample on an agarose gel.arrow_forward
- 5. We have 100 mL of 5X TBE (Tris/Borate/EDTA) solution and need to make 1 L of 2X solution. Solid compounds also exist for each of these materials. The molecular weight of Tris is 157.60; Borate has molecular weight of 58.80 and EDTA has a molecular weight of 292.44. a) What is the stock concentration? b) What is the final concentration? c) What is the starting volume? d) What is the final volume? e) Write out the equation used to solve this. Show all your work. Are we able to make a solution using this dilution method using are starting materials? If not, propose another way to make the desired concentration and volume of the solution. Show your work.arrow_forwardWhat is the pH of a 0.1 M phosphate buffer (pKa = 6.86) that contains 10 times more of the conjugate acid form than the conjugate base form? a.) 5.86 b.) 16.86 c.) -3.14 d.) 6.86arrow_forwardA patient’s treatment plan requires two IV solutions to be administered:20% magnesium sulfate, MgSO4, and 10% calcium chloride, CaCl2. Thenurse is concerned, MgSO4 and CaCl2 are listed as incompatible IVsolutions with the risk of solid precipitate formation (image shown toright). Write the chemical equation that predicts the products thatform when combining MgSO4 and CaCl2. Please use the providedsolubility chart to help you determine chemical phases.arrow_forward
- Draw titration curve for compound X when NaOH equivalence(s) is/are added. Indicate the buffering region(s) with a box.arrow_forwardLipids from an organic sample are extracted separately by column chromatography using the ff eluants: 1st eluant - chloroform: methanol: water (6:3:1) 2nd eluant - methanol: chloroform (9:1) 3rd eluant – petroleum ether: ethyl ether (1:1) Test results for each eluate are tabulated below. Identify the possible lipid present in each eluate. Test Reagents 1. KHSO4 2. HNO3,(NH4)3MoO4 3. CHCl3, acetic anhydride, conc. H2SO4 4. -naphthol, conc. H2SO4 5. Hydroxylamine HCl, FeCl3 6. ninhydrin reagent POSSIBLE LIPIDS: Triacylglycerol, cholesterol, sphingomyelin, lecithinarrow_forwardHow many grams of HCl are needed to make 10 uL of a 0.005g/mL acid solution?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON