
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
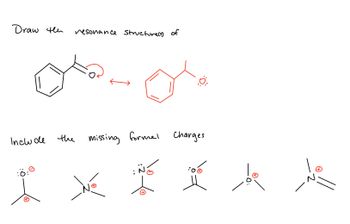
Transcribed Image Text:Draw th
Include
resonance Structure(s) of
the
Ö:
missing formel Charges
:Ö:0
I X I Ik de
Expert Solution
arrow_forward
Step 1

Step by stepSolved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Re-order each list of elements in the table below, if necessary, so that the elements are listed in order of decreasing electronegativity. elements As, S, Se F, I, Br elements in order of decreasing electronegativity Ú 0,0,... X 3arrow_forwardCopy of In the correct Lewis Structure for N2F2, skeletor FNN F, how many lone pairs (unshared pairs) are in the entire molecule? Put a zero formal charge on all toms. You can use my 'simple, quick' method for molecules with only H.C,N,O and F O 1 O 2 O 3 4 O 5 0 8arrow_forwardin each ionic compound. Part C Cal2 Ca o Ca :: 2+ O Ca Submit Previous Answers Request Answer X Incorrect; Try Again; 5 attempts remaining Only covalent honds are drawn using single, double, and triple lines in their Lewis symbol because they share electrons. lonic bonds do not share electrons 9:02 PM A U O G 4 4/21/2021arrow_forward
- Write Lewis structures for the following molecules or ions, which have central atoms that do not obey the octet rule:arrow_forward4 Which of the following compounds would you expect to have the greatest difference in electronegativity between the two elements? tab hift O LIF O IF O Lil esc caps lock O CsF OLICS O Csl A control ! 1 Q A @ 2 N W S ** # 3 X E H 1 option command D $ 4 C R % 5 F LL M T V MacBook Pro ◆ ^ 6 G Y B & N 7 H U * N 8 J ( - 9 ī M K MOSISO O ) 0 V L comarrow_forwardIn the given ion given, what is the formal charge of nitrogen, carbon, and sulfur, respectively? [:N-C=S:]" a. -2, +1, and O. b. 0, -2, and +1. c. -2, 0, and +1. d. +1, 0, and -2. a O b O darrow_forward
- Nonearrow_forwardWhich of the following is not a resonance structure of the species shown L IL ON O "l O III 01 NH₂ NH₂ III IV.arrow_forwardNitrite (NO2−) is an important nutrient in the eutrophic zone of the ocean. Which of the following is the correct set of resonance structures for this ion? Select one: a. IV b. III c. I d. IIarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY