
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
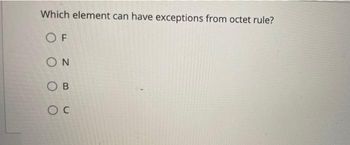
Transcribed Image Text:Which element can have exceptions from octet rule?
OF
ΟΝ
OB
OC
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw structures for both pleasearrow_forwardThe greatest degree of ionic character is anticipated for the bond between: * H and C H and Br Hand Cl ) Br and CIarrow_forwardWrite Lewis structures for the following molecules or ions, which have central atoms that do not obey the octet rule:arrow_forward
- From the Lewis structures of the species given, pick all of those in which the central atom obeys the octet rule. :ci: :ci: ci: .. :CI: H-C-H H H-B-H None of the Above HI :o:arrow_forwardSelect the correct Lewis structure for the following compound. rof. H—O s—O—H HO OF SOO H :0: អះចំនះÖH :0: H ៖ ម្ល៉េះនះ H :0: :0: Ho: so H :0: :0: H: s: H :O: : 0: ….arrow_forwardThe specific heat of aluminum is 0.214 cal/g. c. Determine the energy, in calories, necessary to raise the temperature of a 55.5 g piece of aluminum from 23.0 to 48.6°C. A. 109 cal B. 273 cal C. 577 cal D. 347 cal E. 304 cal of reactions can be Standard enthalpy calculated from standard enthalpies of formation of reactants. A. True B. Falsearrow_forward
- Hydrochloric acid molecule holds together by a(n) O ionic bond polar covalent bond double bond nonpolar bond none of thesearrow_forwardMolecule Lewis Diagram HNO H₂CO 3D Diagram Name of VSEPR shapearrow_forwardDraw the Lewis Structure of BF3 Molecular Geometry? Ideal Bond Angles? Are there Polar Bonds Present (Indicate on Lewis do structure Is there an Overall Dipole? Octet rule violator and how?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY