
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
1. What is wrong with this Lewis structure? The shape is not of concern.
2. Label the formal charges on each atom in each resonance structure below.
![### Question: Analyzing Lewis Structures
#### Problem 1:
**Question:**
What is wrong with this Lewis structure? The shape is not of concern.
**Image Description:**
The Lewis structure consists of three carbon atoms connected in a chain. The first carbon is bonded to three hydrogen atoms, the second carbon has a double bond with the third carbon and a single bond with a hydrogen atom. The third carbon is bonded with a hydrogen atom.
**Notes:**
Analyze the bonds and the valence electrons of each atom to determine any inaccuracies.
#### Problem 2:
**Question:**
Label the formal charges on each atom in each resonance structure below.
**Image Description:**
Resonance structure diagram showing a compound with a sequence of atoms and possible resonance forms.
**Instructions:**
Click on "Show Your Work" to proceed with labeling the formal charges on each atom depicted in the resonance structures provided. Pay close attention to electron distribution and ensure appropriate calculations are made for formal charges.
**Note:**
Formal charge calculations are based on the formula:
\[ \text{Formal Charge} = \text{Valence Electrons} - \text{Non-bonding Electrons} - \frac{\text{Bonding Electrons}}{2} \]](https://content.bartleby.com/qna-images/question/2b52cd77-b3d2-4b5d-8903-b23362bef087/c12582c1-1ecc-4aeb-8d7c-1825e6128b96/1553yy6_thumbnail.png)
Transcribed Image Text:### Question: Analyzing Lewis Structures
#### Problem 1:
**Question:**
What is wrong with this Lewis structure? The shape is not of concern.
**Image Description:**
The Lewis structure consists of three carbon atoms connected in a chain. The first carbon is bonded to three hydrogen atoms, the second carbon has a double bond with the third carbon and a single bond with a hydrogen atom. The third carbon is bonded with a hydrogen atom.
**Notes:**
Analyze the bonds and the valence electrons of each atom to determine any inaccuracies.
#### Problem 2:
**Question:**
Label the formal charges on each atom in each resonance structure below.
**Image Description:**
Resonance structure diagram showing a compound with a sequence of atoms and possible resonance forms.
**Instructions:**
Click on "Show Your Work" to proceed with labeling the formal charges on each atom depicted in the resonance structures provided. Pay close attention to electron distribution and ensure appropriate calculations are made for formal charges.
**Note:**
Formal charge calculations are based on the formula:
\[ \text{Formal Charge} = \text{Valence Electrons} - \text{Non-bonding Electrons} - \frac{\text{Bonding Electrons}}{2} \]
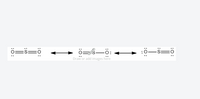
Transcribed Image Text:### Resonance Structures of Sulfur Dioxide (SO₂)
In the study of sulfur dioxide (SO₂), it is crucial to understand the concept of resonance structures. Resonance structures are different Lewis structures that represent the same molecule. They illustrate the delocalization of electrons within the molecule. Here, we present the resonance structures of sulfur dioxide.
**Diagram Description:**
The image demonstrates three resonance structures of the SO₂ molecule with arrows indicating the resonance between the structures:
1. **First Resonance Structure:**
- The sulfur atom (S) is double-bonded to two oxygen atoms (O).
- The sulfur atom bears a formal charge of zero.
- Each oxygen atom has four lone electrons (two lone pairs), with the usual formal charge of zero.
2. **Double Arrow Explanation:**
- A double-headed arrow between the structures indicates that these are resonance forms of the same molecule.
- This signifies that the actual structure of the molecule is a hybrid of these forms, with the delocalized electrons contributing to the overall stability of the molecule.
3. **Second Resonance Structure:**
- One oxygen atom forms a double bond with sulfur, while the other oxygen forms a single bond with sulfur and carries a negative formal charge.
- The sulfur atom in this structure has a positive formal charge, due to the lack of electron density compared to the first structure.
- The single-bonded oxygen has six lone electrons (three lone pairs), and the double-bonded oxygen has four lone electrons (two lone pairs).
4. **Third Resonance Structure:**
- Similar to the second structure but with the positions of the single and double bonds reversed.
- One oxygen atom forms a single bond with sulfur and carries a negative formal charge.
- The sulfur atom has a positive formal charge, as in the second structure.
- The single-bonded oxygen has six lone electrons (three lone pairs), and the double-bonded oxygen has four lone electrons (two lone pairs).
**Significance:**
The resonance structures indicate that the electron pair (or lone pair) on the sulfur atom can be delocalized across the molecule, resulting in partial double-bond character for both S-O bonds. This delocalization increases the stability of the molecule.
In essence, neither of the individual resonance structures fully or accurately depicts the actual bonding in SO₂; rather, the actual structure is a resonance hybrid of all
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The formal charge is the "charge" an element would have in a molecule or ion if all of the bonding electrons were shared equally between atoms. We can draw three inequivalent Lewis structures for the sulfite ion, SO3²-. The concepts of formal charge and electronegativity can help us choose the structure that is the best representation. 1. Assign formal charges to the elements in each of the structures below. Note: Count oxygen atoms starting from the left for each structure. Formal Charge S 01 Use the References to access important values if needed for this question. 02 03 S KNENEN :0: B A I 2- 2. The best Lewis structure for SO3²- is A :0: C Previous Nextarrow_forwardDetermine whether a molecule will have one or more resonance structures. Guiacol is a molecule responsible for a vanilla aroma. Will this molecule have resonance structures? Explain. A structural formula is provided here: C6H4(OH)(OCH3) and a structure is shown below. H. H H.arrow_forwardThe formal charge is the "charge" an element would have in a molecule or ion if all of the bonding electrons were shared equally between atoms. We can draw three inequivalent Lewis structures for carbon dioxide , CO2 . The concepts of formal charge and electronegativity can help us choose the structure that is the most significant representation.1. Assign formal charges to the elements in each of the structures below. A B C Formal ChargeO1CO2 2. The structure that contributes most significantly to the overall electronic structure of CO2 is (A,B, or C) .arrow_forward
- Draw the Lewis structure (with formal charges) of the indicated chemical species. If there is resonance, show all possible resonance structures. 5. CH3CN 6. NH2Brarrow_forwardDraw the lewis structure with formal charges. If the lewis structure has resonance please show all possible resonance structures. 4. SO3arrow_forwardraw the Lewis structure of Cl,02 based on the skeletal structure. Do not add formal charges.arrow_forward
- Draw the BEST lewis structure (with formal charges) of the indicated chemical species. (Draw all possible resonance structures). 9. HSeO4^- 10. HIO2arrow_forwardDraw the lewis structure with formal charges. If the lewis structure has resonance please show all possible resonance structures. 2. CINO2 (N is the central atom)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY