
General, Organic, and Biological Chemistry
7th Edition
ISBN: 9781285853918
Author: H. Stephen Stoker
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
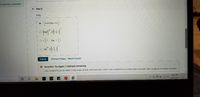
Transcribed Image Text:in each ionic compound.
Part C
Cal2
Ca
o Ca ::
2+
O Ca
Submit
Previous Answers Request Answer
X Incorrect; Try Again; 5 attempts remaining
Only covalent honds are drawn using single, double, and triple lines in their Lewis symbol because they share electrons. lonic bonds do not share electrons
9:02 PM
A U O G 4
4/21/2021
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The percent ionic character of a bond can be approximated by the formula 16+3.52 , where is the magnitude of the difference in the electronegativities of the atoms (see Fig. 3.18). Calculate the percent ionic character of HF, HCl, HBr, HI, and CsF, and compare the results with those in Table 3.7.arrow_forwardFor each of the following molecular models, write an appropriate Lewis formula.arrow_forwardOn the basis of the electronegativity values given in Fig. 12.3, indicate whether each of the following bonds would be expected to be ionic, covalent, or polar covalent. msp;a.HOc.HHb.OOd.HClarrow_forward
- A stable triatomic molecule can be formed that contains one atom each of nitrogen, sulfur, and fluorine. Three bonding structures are possible, depending on which is the central atom: NSF, SNF, and SFN. (a) Write a Lewis diagram for each of these molecules, indicating the formal charge on each atom. (b) Often, the structure with the least separation of formal charge is the most stable. Is this statement consistent with the observed structure for this molecule—namely, NSF, which has a central sulfur atom? (c) Does consideration of the electronegativities of N, S, and F from Figure 3.18 help rationalize this observed structure? Explain.arrow_forwardThe valence shell of an atom in a legitimate Lewis structure (see Figure 2.3) has what in commonwith the valence shell of a noble gas? (Noble gases are stable elements found in the last column ofthe periodic table, e.g., He, Ne, Ar, etc.)arrow_forwardO 14-C O Quiz: O 17-Pc PE JPG to M Inbox 14-C O Calen 13-Cc On a O 16-M New Tab com /courses/2126119/files/171672875?module_item_id=55281625 377 > Files > 15-Lewis Structures.pdf 15-Lewis Structures.pdf Download 15-Lewis Structures.pdf (88.1 KB) Draw a valid Lewis structure for each compound, using the given arrangement of atoms. (May need multiple bonds) 1. HCN N 2. CH,0 H H. 99+ 58°F Coparrow_forward
- Step 4arrow_forwarded Lewis struc :: :Z: : Z: |d=8: the proposed Lewis structure reasonable? Yes. No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* Yes. No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* Yes. No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:*arrow_forwardAmong the Lewis structures below which one(s) is(are) not correct? Multiple choice allowed HOI HC. C ICI I H H -I H O-I H HCH ΤΗ CH C H C-H H I H..N N 12=0 H Н. H 10-C=NIarrow_forward
- aortunity, Less Debt | Be X Student Home OMail-Shelby Hamrington-Outlo X VO Chem 101 valence electrons of carbon- Bir X A https://app.101edu.co Question 37 of 48 A Lewis structure with placeholder central atom is shown below. If the charge of the molecule is -1, choose the possible identity or identities of the central atom. A) F B) N, P, or As C) C, Si, or Ge D) B or Al E) CI, Br, or I archarrow_forward< : ed Lewis structure N 0_C_0: H = Br O: the proposed Lewis str Yes. No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are: * Yes. No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* Yes. No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:*arrow_forwardDecide whether these proposed Lewis structures are reasonable. proposed Lewis structure .. :Z: || :Z: H = Br N101 C1: O оо Is the proposed Lewis structure reasonable? O Yes. No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* Yes. No, it has the wrong number of valence electrons. The correct number is: O Yes. No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* * If two or more atoms of the same element don't satisfy the octet rule, just enter the chemical symbol as many times as necessary. For example, if two oxygen atoms don't satisfy the octet rule, enter "O,0".arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning


Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning