
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN: 9781305079250
Author: Mark S. Cracolice, Ed Peters
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Step 4
Transcribed Image Text:C CICC C Clear A C (Refer C My Ar
C Create C Home C Tor-a
C EXERC
CI Chec
O8 https://east.cengagenow.com/ilrn/takeAssignment/takeCovalentActivity.do?locator=assignment-take
Clear
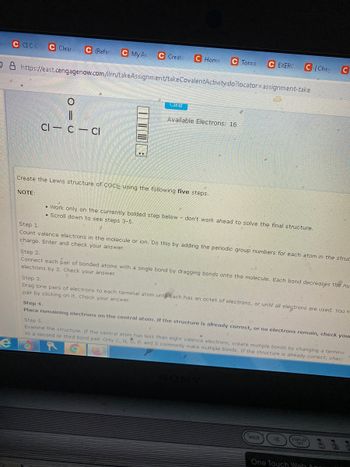
Available Electrons: 16
||
Cl- c-cl
Create the Lewis structure of COCl2 using the following five steps.
NOTE:
• Work only on the currently bolded step below don't work ahead to solve the final structure.
• Scroll down to see steps 3-5.
Step 1.
Count valence electrons in the molecule or ion. Do this by adding the periodic group numbers for each atom in the struc
charge. Enter and check your answer.
Step 2.
Connect each pair of bonded atoms with a single bond by dragging bonds onto the molecule. Each bond decreases the nu
electrons by 2. Check your answer.
Step 3.
Drag lone pairs of electrons to each terminal atom unt each has an octet of electrons, or until all electrons are used. You m
pair by clicking on it. Check your answer.
Step 4.
Place remaining electrons on the central atom. If the structure is already correct, or no electrons remain, check your
Step 5.
Examine the structure. If the central atom has less than eight valence electrons, create multiple bonds by changing a terminal
to a second or third bond pair. Only C, N, O, P, and S commonly make multiple bonds. If the structure is already correct, check
e
WEB
d
DISPLAY
One Touch Web De
O C
C
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A stable triatomic molecule can be formed that contains one atom each of nitrogen, sulfur, and fluorine. Three bonding structures are possible, depending on which is the central atom: NSF, SNF, and SFN. (a) Write a Lewis diagram for each of these molecules, indicating the formal charge on each atom. (b) Often, the structure with the least separation of formal charge is the most stable. Is this statement consistent with the observed structure for this molecule—namely, NSF, which has a central sulfur atom? (c) Does consideration of the electronegativities of N, S, and F from Figure 3.18 help rationalize this observed structure? Explain.arrow_forwardTwo possible Lewis diagrams for sulfine (H2CSO) are (a) Compute the formal charges on all atoms. (b) Draw a Lewis diagram for which all the atoms in sulfine have formal charges of zero.arrow_forwardBased on the concept of formal charge, what is the central atom in (a) HCN (do not include H as a possibility)? b) NOCI (Cl is always a terminal atom)?arrow_forward
- For each of the following bonds, draw a figure indicating the direction of the bond dipole, including which and of the bond is positive and which is negative. msp;a.CFc.COb.SiCd.BCarrow_forwardStep 4arrow_forwardN MyLab and Mastering Course Home b Search results for "Which of the At Electronegativity Table of the Ele x + A openvellum.ecollege.com/course.html?courseld=16418591&OpenVellumHMAC=fd2ce09746a2194453c324a109d1a8ec#10001 Scores Part A Pearson eText Part B Study Area Indicate the more electronegative atom in each pair. Match the atoms in the left column to the appropriate blanks in the sentences on the right. Document Sharing User Settings Reset Help Course Tools > B Given the bond P-N, the more electronegative atom in the bond is Given the bond B-F, the more electronegative atom in the bond is Br Given the bond H-Br, the more electronegative atom in the bond is H P N Submit Request Answer P Pearson Copyright © 2021 Pearson Education Inc. All rights reserved. | Terms of Use | Privacy Policy. | Permissions | Contact Us | 2:55 AM P Type here to search 4/14/2021arrow_forward
- Assign formal charges to each atom in the resonance form for SOCI2 given below. :o: .. .. :cl- -s-cl: .. O O for CI, +1 for S, and -1 for O O -1 for CI, +4 for S, and -2 for O O O for CI, 0 for S, and 0 for O O -1 for Cl, -2 for S, and -2 for Oarrow_forward2. each atom if it has. Show all steps (NHSB). Draw the Lewis structures for the following molecules. Indicate the formal charge on (a) N,²- (b) SO3 olda ad ni d Two molecules may both be correctly described as "bent" even though one has a bond angle of 118° and the other has a bond angle of 105°. How is this possible?arrow_forwardvhich of the following pairs of Lewis structures is/are resonance structures of one another? :O: 25. . O.. aniio.0-cEN: o=c=N alav sr ai teriW (2tni %3D0. :0-CEN OLx 10. O. Corx E.E (5 II III I c) Il and III d) I, II, and II a) I only b) II onlyarrow_forward
- help me fill out the table.arrow_forwardO 14-C O Quiz: O 17-Pc PE JPG to M Inbox 14-C O Calen 13-Cc On a O 16-M New Tab com /courses/2126119/files/171672875?module_item_id=55281625 377 > Files > 15-Lewis Structures.pdf 15-Lewis Structures.pdf Download 15-Lewis Structures.pdf (88.1 KB) Draw a valid Lewis structure for each compound, using the given arrangement of atoms. (May need multiple bonds) 1. HCN N 2. CH,0 H H. 99+ 58°F Coparrow_forwardDONT PROVIDE AI solutin....arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning