
Human Anatomy & Physiology (11th Edition)
11th Edition
ISBN: 9780134580999
Author: Elaine N. Marieb, Katja N. Hoehn
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
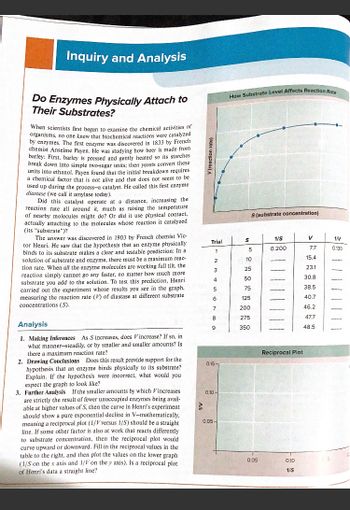
Transcribed Image Text:**Inquiry and Analysis: Do Enzymes Physically Attach to Their Substrates?**
When scientists first began to examine the chemical activities of organisms, no one knew that biochemical reactions were catalyzed by enzymes. The first enzyme was discovered in 1833 by French chemist Anselme Payen. He was studying how beer is made from barley: First, barley is pressed and gently heated so its starches break down into simple two-sugar units; then yeasts convert these units into ethanol. Payen found that the initial breakdown requires a chemical factor that is not alive and that does not seem to be used up during the process—a catalyst. He called this first enzyme diastase (we call it amylase today).
Did this catalyst operate at a distance, increasing the reaction rate all around it, much as raising the temperature of nearby molecules might do? Or did it use physical contact, actually attaching to the molecules whose reaction it catalyzed (its “substrate”)?
The answer was discovered in 1903 by French chemist Victor Henri. He saw that the hypothesis that an enzyme physically binds to its substrate makes a clear and testable prediction: In a solution of substrate and enzyme, there must be a maximum reaction rate. When all the enzyme molecules are working full tilt, the reaction simply cannot go any faster, no matter how much more substrate you add to the solution. To test this prediction, Henri carried out the experiment whose results you see in the graph, measuring the reaction rate (V) of diastase at different substrate concentrations (S).
**Analysis**
1. **Making Inferences**: As S increases, does V increase? If so, in what manner—steadily, or by smaller and smaller amounts? Is there a maximum reaction rate?
2. **Drawing Conclusions**: Does this result provide support for the hypothesis that an enzyme binds physically to its substrate? Explain. If the hypothesis were incorrect, what would you expect the graph to look like?
3. **Further Analysis**: If the smaller amounts by which V increases are strictly the result of fewer unoccupied enzymes being available at higher values of S, then the curve in Henri’s experiment should show a pure exponential decline in V—mathematically, meaning a reciprocal plot (1/V versus 1/S) should be a straight line. If some other factor is also at work that reacts differently to substrate concentration, then the reciprocal plot would curve upward or downward.
Expert Solution
arrow_forward
Step 1
Enzyme are usually protein molecules that are highly specific to their substrate. These are biological catalyst as they catalyse a particular reaction without being itself used up in the reaction. These increase the rate of a reaction.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Similar questions
- BIOCHEMISTRY QUESTION The initial rate (VO) data as a function of substrate concentration [S] for an enzyme (Enzyme1) that obeys Michaelis-Menten kinetics are shown in the table below. The total enzyme concentration is 2.5 nM. [S](μm) 16.0 8.0 4.0 2.0 1.0 v (μMsec ¹) 48.5 32.7 19.8 11.1 5.88 (a) Calculate KM for the enzyme, using the estimated slope (slope = (y2-y1)/(x2-x1)) from a Lineweaver-Burk plot. Show your work and don't forget the units! (b) When the enzyme concentration is 2.5 nM, a Lineweaver-Burk plot of this data gives a line with a y- intercept of 0.0106 μ M-1 sec. Calculate kcat for the enzyme. (Show your work and don't forget to include units). (c) A second enzyme (Enzyme2) also obeys Michaelis-Menten kinetics. Its kcat and KM are 800 sec-1 and 5 µM, respectively. Which is the more efficient enzyme? Briefly explain d) Is Enzyme 1 diffusion limited? Explain.arrow_forwardExplain the difference between air-cooled and ice cooled enzyme as both had been pre-heated but one showed a bit more activity than the otherarrow_forwardPart 1: Assess the following partial results section below by editing it for brevity by omitting any unnecessary parts (1 point), explain why you decided to remove certain sections (1 point): To evaluate inhibitory effects of the selected molecules, 10mM stock solutions of each molecule were prepared in DMSO. A reaction mixture (200μl) was prepared with the same formula optimized for the enzyme activity assay (0.1 M Tris-HCl ph 8, 0.1 M KCI, 25 mM NaCl, 0.25 mM ATP, and two units of inorganic yeast pyrophosphatase) with 10 µM of the sample molecule. The reaction mixture was incubated for 20 minutes at ambient temperature. Enzymatic reaction was triggered by addition of the substrate B (0.2 mM) and the absorbance of the product was monitored at 290 nm for 10 minutes. Six out of 15 sample molecules showed appreciable inhibition at 10 μM (Figure 5). Three of the molecules, A3, A6, and A7 exhibited more than 50% inhibition of the enzyme activity and were further diluted to find the minimal…arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON
Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education,
Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education, Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company
Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co.
Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co. Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education
Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education

Human Anatomy & Physiology (11th Edition)
Biology
ISBN:9780134580999
Author:Elaine N. Marieb, Katja N. Hoehn
Publisher:PEARSON

Biology 2e
Biology
ISBN:9781947172517
Author:Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:OpenStax

Anatomy & Physiology
Biology
ISBN:9781259398629
Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa Stouter
Publisher:Mcgraw Hill Education,

Molecular Biology of the Cell (Sixth Edition)
Biology
ISBN:9780815344322
Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter Walter
Publisher:W. W. Norton & Company

Laboratory Manual For Human Anatomy & Physiology
Biology
ISBN:9781260159363
Author:Martin, Terry R., Prentice-craver, Cynthia
Publisher:McGraw-Hill Publishing Co.

Inquiry Into Life (16th Edition)
Biology
ISBN:9781260231700
Author:Sylvia S. Mader, Michael Windelspecht
Publisher:McGraw Hill Education