Beakers #: 2 Contents in beakers 10 mL of 0.5 M HC:H;O 8 mL of 0.5 M HCSH302 6 mL of 0.5 M HC5H302 + 2 mL of 0.5 M NaC2H3O2 4 mL of 0.5 M NaC2H3O2 4 mL of 0.5 M HC5H302 + 6 mL of 0.5 M NaC2H302 5 2 mL of 0.5 M HC5H302 + 8 mL of 0.5 M NaC2H302 6 10 mL of 0.5 M NaC2H302 part 1 pH (Buffer 2.66 3.80 4.50 4.85 5.35 7.71 preparations) Part 2pH 1.56 3.23 4.00 4.00 4.83 (Strong Acid (5 drops) (5 drops) (12 drops) (15 drops) (7 drops) 5.90 (5 drops) added) Part 3 pH 3.83 4.38 4.99 5.40 5.98 (Strong Base (5 drops) (5 drops) (10 drops) (10 drops) (5 drops) 12.73 (5 drops) added)
Beakers #: 2 Contents in beakers 10 mL of 0.5 M HC:H;O 8 mL of 0.5 M HCSH302 6 mL of 0.5 M HC5H302 + 2 mL of 0.5 M NaC2H3O2 4 mL of 0.5 M NaC2H3O2 4 mL of 0.5 M HC5H302 + 6 mL of 0.5 M NaC2H302 5 2 mL of 0.5 M HC5H302 + 8 mL of 0.5 M NaC2H302 6 10 mL of 0.5 M NaC2H302 part 1 pH (Buffer 2.66 3.80 4.50 4.85 5.35 7.71 preparations) Part 2pH 1.56 3.23 4.00 4.00 4.83 (Strong Acid (5 drops) (5 drops) (12 drops) (15 drops) (7 drops) 5.90 (5 drops) added) Part 3 pH 3.83 4.38 4.99 5.40 5.98 (Strong Base (5 drops) (5 drops) (10 drops) (10 drops) (5 drops) 12.73 (5 drops) added)
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.21QAP
Related questions
Question
using the table below can you help me calculate
A) the concentration of weak acid (HC2H302) using (volume of 0.5 ml HC2H302 × 0.5 / total volume of solution)
B) the concentration if conjugate base ( C2H302) using (volume of 0.5 mL NaC2H302 x 0.5 / total volume of solution)
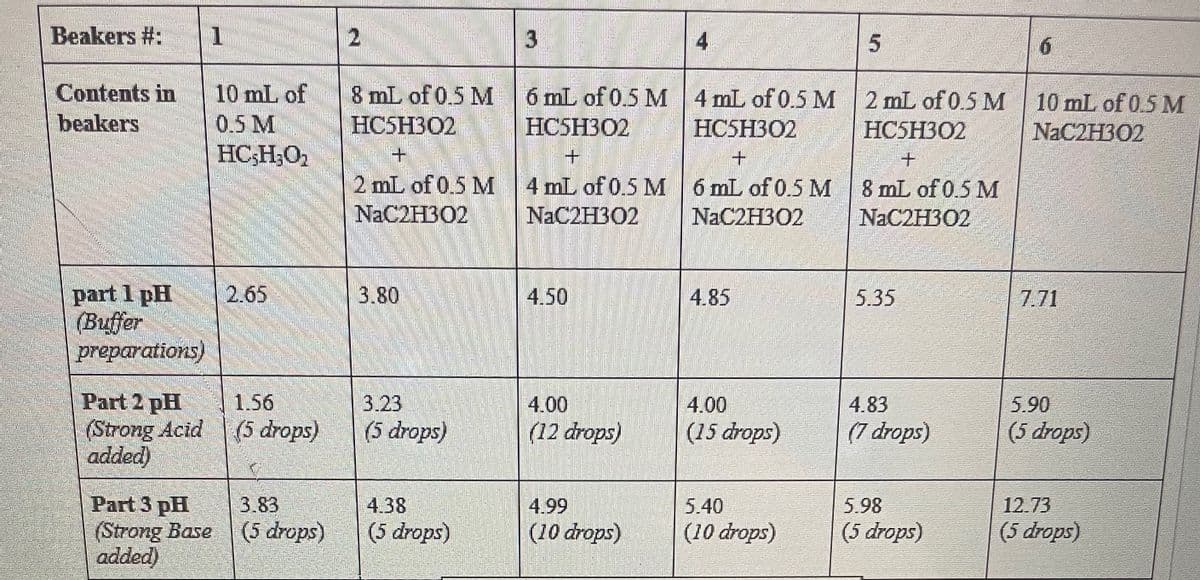
Transcribed Image Text:Beakers #:
2
Contents in
beakers
10 mL of
0.5 M
HC:H;O
8 mL of 0.5 M
HCSH302
6 mL of 0.5 M
HC5H302
+
2 mL of 0.5 M
NaC2H3O2
4 mL of 0.5 M
NaC2H3O2
4 mL of 0.5 M
HC5H302
+
6 mL of 0.5 M
NaC2H302
5
2 mL of 0.5 M
HC5H302
+
8 mL of 0.5 M
NaC2H302
6
10 mL of 0.5 M
NaC2H302
part 1 pH
(Buffer
2.66
3.80
4.50
4.85
5.35
7.71
preparations)
Part 2pH
1.56
3.23
4.00
4.00
4.83
(Strong Acid
(5 drops)
(5 drops)
(12 drops)
(15 drops)
(7 drops)
5.90
(5 drops)
added)
Part 3 pH
3.83
4.38
4.99
5.40
5.98
(Strong Base
(5 drops)
(5 drops)
(10 drops)
(10 drops)
(5 drops)
12.73
(5 drops)
added)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you







Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning