
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
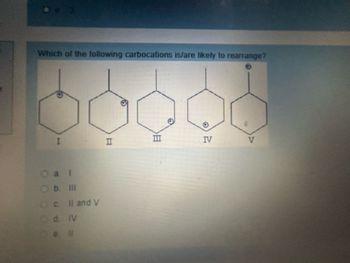
Transcribed Image Text:Which of the following carbocations is/are likely to rearrange?
IV
I
II
Ob. III
Oc II and V
Od IV
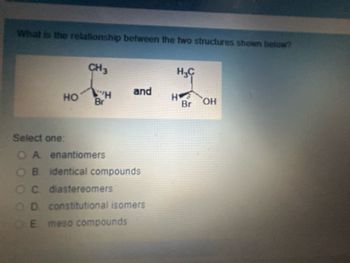
Transcribed Image Text:What is the relationship between the two structures shown below?
CH3
HC
and
HO
H
H
Br
Br OH
Select one:
OA
enantiomers
OB. identical compounds
OC. diastereomers
OD
constitutional isomers
E
meso compounds
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- Which carbocation would be least stable? II + + III IV Varrow_forward15. Which of the following carbocations is the most stable? a) ethyl carbocation, CH3CH2+ b) rpropyl carbocation, CH3CH2CH2* c) Isopropyl carbocation, (CH3)2CH* d) t-butyl carbocation, (CH3)3C+arrow_forwardWhich of the following carbocations is the most stable? A B D A B MeO C O₂N Darrow_forward
- Please don't provide handwritten solution....arrow_forwardIn light of your answer to Problem 11-74, explain why one of the following isomers undergoes E2 reaction approximately 100 times as fast as the other. Which isomer is more reactive, and why?arrow_forward2. What is the outcome of the reaction shown below? SH O=ZO Catalytic Et3Narrow_forward
- 7.+ 8.arrow_forwardWhich compound(s) will be fully deprotonated (>99%) by reaction with one molar equivalent of sodium hydroxide? I, II, III I, || I, III I only II, III SH | H3C-C=C-H || III NH2arrow_forwardThe reaction shown proceeds via a single transition state with a trigonal bipyramidal geometry. C1 C2 Br: + H3C 0: Two curved arrows are required to indicate all of the bond-making and bond-breaking processes in this reaction. Where should one of the arrows be drawn, if CH3O is the nucleophile? from a Br LP to the O atom from C1 to the O atom from C2 to the O atom from the C-O bond to Br from an O LP to C1 from an O LP to C2arrow_forward
- 2. Show the mechanism with arrow formalism to show the formation of the most stable product. Which of the following terms apply to the mechanism(s) i) Regioselectivity (ii)stereoselectivity (iii)stereospecificity. Explain H+ (aq) from sulfuric acid NaCN most stable productarrow_forwardKl117.arrow_forwardThe cis and trans 2-butene stereoisomers have the following rate constant values associated with the electrophilic addition of water in an acid medium at the same temperature. what is the difference between the two values through the energy profile of each reaction. CH3 H,O productos H+ H3C k = 3.51 x 10- M's1 H3C CH3 H,0 > productos H+ k = 8.32 x 10®M's1arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
