
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
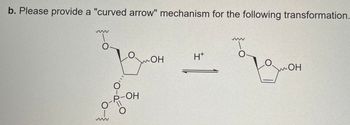
Transcribed Image Text:b. Please provide a "curved arrow" mechanism for the following transformation.
OH
H+
Браон
-OH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Provide an arrow pushing mechanism for the following transformation. Draw out a representation of the transition-state in the rate-determining step.arrow_forwardProvide a detailed, stepwise mechanism for the reaction shown below. CH3OH CH3 heat CH3arrow_forward3. Provide a complete arrow pushing mechanism for the following transformation. H b HO NaOH heatarrow_forward
- Draw a mechanism for the following transformation. [H,SO4] cat. HO.arrow_forwardQ2. When (CH3CH2)3CBR is added to CH3OH at room temperature, the major product is (CH30)C(CH2CH3)3 and a minor product is CH3CH3DC(CH2CH3)2. Propose a mechanism for the product that is formed by the substitution reaction. Use curved arrows to show the movement of electrons.arrow_forwardQ4. The herbicide oxyfluorfen can be prepared by the reaction between phenol and an aryl fluoride. Propose a curved arrow mechanism for this reaction. 1 F3C. LOCH2CH3 F3C. КОН CI `NO2 CI OH LOCH2CH3 `NO2 Oxyfluorfenarrow_forward
- Fill in the missing reagents a-e in the following scheme:arrow_forwardProvide a complete curved-arrow mechanism for this transformation. I am confused.arrow_forwardProvide a mechanism for the following transformation, clearly illustrating each step with curved arrows. CH3 conc. H₂SO4 A CH3arrow_forward
- Draw the major organic product of the reaction. Indicate the stereochemistry via wedge/dash bonds, including explicit H and D atoms, at the stereogenic center. Omit byproducts such as salts or methanol. Br NaOCH CH OH Harrow_forwardDraw the step-by-step mechanism using curved arrows of the following reaction.arrow_forward5. Which bromocyclohexane starting material would react faster - the cis or trans, and explain why? Draw both chair forms of the starting material to prove your point. Br K* OC(CH) K* OC(CH); Br cis transarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
