
World of Chemistry, 3rd edition
3rd Edition
ISBN: 9781133109655
Author: Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher: Brooks / Cole / Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Show a complete synthesis of the following molecule starting with cyclohexane (as many times as you wish), any molecules of three (3) carbons or fewer, and any other reagents.
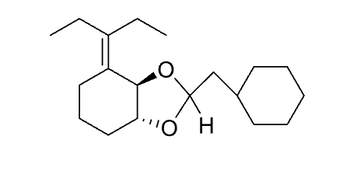
Transcribed Image Text:Η Ο
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- I- Select all of the molecules to the right that are alkenes. H. H-C-C-H - H H C C-Harrow_forwardQuestion 22 Which of the following Lewis structures will have resonance forms? O || : 0: N- CIarrow_forwardSome solar-heated homes use large beds of rocks to store heat. (a) How much heat is absorbed by 100.0 kg of rocks if their temperature increases by 12C? (Assume that c=0.82J/gC.) (b) Assume that the rock pile has total surface area 2 m2. At maximum intensity near the earth's surface, solar power is about 170 watts/m2. (1watt=1J/s.) How many minutes will it take for solar power to produce the 12C increase in part (a)?arrow_forward
- 5735112480241329813180832311&eISBN=9781305862883&id=1774598910&snapshotid=33... A bomb calorimeter, or constant volume calorimeter, is a device often used to determine the heat of combustion of fuels and the energy content of foods. Since the "bomb" itself can absorb energy, a separate experiment is needed to determine the heat capacity of the calorimeter. This is known as calibrating the calorimeter. In the laboratory a student burns a 0.284-g sample of phenanthrene (C14H10) in a bomb calorimeter containing 1020. g of water. The temperature increases from 24.60 °C to 26.80 °C. The heat capacity of water is 4.184 J g-¹°C-¹. The molar heat of combustion is -7054 kJ per mole of phenanthrene. C14H10(s) + 33/2 O₂(g) 14 CO₂(g) + 5 H₂O(l) + Energy Calculate the heat capacity of the calorimeter. heat capacity of calorimeter = An error has been detected in your answer. Check for typos, miscalculations etc. before submitting your answer. b Calorimeter Calculations: This is group attempt 1 of 10…arrow_forwardΝΗ Heat ?arrow_forward8. What is the symbol for energy/heat flow in the field of thermochemistry? O Hf E Harrow_forward
- What is the magnitude of the uncertainty introduced by the lack of knowledge of the specific heat samplearrow_forwardWhich has the greater kinetic energy, Object 1 with a mass of 2.9 kg and a velocity of 0.71 m/s or Object 2 with a mass of 1.3 kg and a velocity of 2.2 m/s? KE of Object 1 = J KE of Object 2 =| J |has greater kinetic energy. Object 1 Object 2 Retny Entire Groun O more aroun attempts remainingarrow_forwardPls help me answer 1,5 and 8. Thank youarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning