
Chemistry
9th Edition
ISBN: 9781133611097
Author: Steven S. Zumdahl
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Chemistry
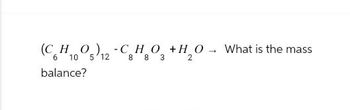
Transcribed Image Text:(C_H_O_) -CHO +H_0
(CoH5)2-CHO
6 10
12
8 8 3
balance?
What is the mass
2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Chlorine exists mainly as two isotopes, 37Cl and 33Cl. Which is more abundant? How do you know?arrow_forwardA sample of an organic compound (a nonelectrolyte) weighing 1.35 g lowered the freezing point of 10.0 of benzene by 3.66 C. Calculate the molar mass of the compound.arrow_forwardA sample of cocaine, C17H21O4N, is diluted with sugar, C12H22O11. When a 1.00-mg sample of this mixture is burned, 1.00 mL of carbon dioxide (d=1.80g/L) is formed. What is the percentage of cocaine in this mixture?arrow_forward
- 4.80 The reaction shown below is used to destroy Freon-12 (CF2Cl2), preventing its release into the atmosphere. What mass of NaF will be formed if 250.0 kg of CF2Cl2 and 400.0 kg of Na2C2O4 are heated and allowed to react to completion? CF2Cl2+2Na2C2O42NaF+2NaCl+C+4CO2arrow_forwardIf you had a mole of U.S. dollar bills and equally distributed the money to all of the people of the world, how rich would every person be? Assume a world population of 7 billion.arrow_forward4. Epsom salt is MgSO4 · 7H2O. When heated to 70 to 80°C it loses some, but not all, of its water of hydration. Suppose you heat 2.465 g of Epsom salt to 75°C and find that the mass is now only 1.744 g. What is the formula of the slightly dehydrated salt? Mg SO4 · 6H2O MgSO4 · 5H2O MgSO4 · 4H2O MgSO4 · 3H2Oarrow_forward
- Boron nitride reacts with iodine monofluoride i trichlorofluoro methane at 30°C to produce pure nitrogen triiodide and by-product (BF3). :math>BN+3IFNI3+BF3 l type='a'> What mass of iodine monofluoride must be used to produce 30.0 g of nitrogen triiodide? When 30.0 g of nitrogen triiodide is produced, what is the maximum mass of by-product (BF3)created?arrow_forwardIn the laboratory you combine 0.125 g of nickel with CO and isolate 0.364 g of Ni(CO)x. What is the value of x?arrow_forwardWhat is the mass of fish, in kilograms, that one would have to consume to obtain a fatal dose of mercury, if the fish contains 30 parts per million of mercury by weight? (Assume that all the mercury from the fish ends up as mercury (II) chloride in the body and that a fatal dose is 0.20 g of HgCl2.) How many pounds of fish is this?arrow_forward
- Consider an iron bar on a balance as shown. As the iron bar rusts, which of the following is true? Explain your answer. a. The balance will read less than 75.0 g. b. The balance will read 75.0 g. c. The balance will read greater than 75.0 g. d. The balance will read greater than 75.0 g, but if the bar is removed, the rust is scraped off, and the bar replaced, the balance will read 75.0 g.arrow_forwardCisplatin, Pt(NH3)2Cl2, is a chemotherapeutic agent that disrupts the growth of DNA. If the current cost of Pt is $1118.0/troy ounce (1troyoz=31.10g), how many grams of cisplatin can you make with three thousand dollars worth of platinum? How many pounds?arrow_forwardSulfuric acid can be prepared starting with the sulfide ore, cuprite (Cu2S). If each S atom in Cu2S leads to one molecule of H2SO4, what is the theoretical yield of H2SO4 from 3.00 kg of Cu2S?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning


Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax


Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning