
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
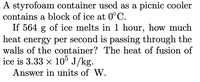
Transcribed Image Text:A styrofoam container used as a picnic cooler
contains a block of ice at 0°C.
If 564 g of ice melts in 1 hour, how much
heat energy per second is passing through the
walls of the container? The heat of fusion of
ice is 3.33 x 10° J/kg.
Answer in units of W.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- At a chemical plant where you are an engineer, a tank contains an unknown liquid. You must determine the liquids specific heat capacity. You put 0.500 kgkg of the liquid into an insulated metal cup of mass 0.200 kgkg. Initially the liquid and cup are at 20∘C∘C. You add 0.500 kgkg of water that has a temperature of 80∘C∘C. After thermal equilibrium has been reached, the final temperature of the two liquids and the cup is 58.2 ∘C∘C. You then empty the cup and repeat the experiment with the same initial temperatures, but this time with 1.00 kgkg of the unknown liquid. The final temperature is 49.4 ∘C∘C. Assume that the specific heat capacities are constant over the temperature range of the experiment and that no heat is lost to the surroundings. a,) Calculate the specific heat capacity of the liquid. b.)Calculate the specific heat capacity of the metal from which the cup is madearrow_forwardA 0.825 kg block of iron, with an average specific heat of 5.60 × 102 J/kg·K, is initially at a temperature of 254.0◦C. The block of iron is placed in a calorimeter with 16.4 g of water at 12.2◦C. What is the final thermal equilibrium temperature? If the answer if 100.0◦C, how much water is still in liquid form? Note: Treat the mass and heat capacity of the calorimeter as neglible.arrow_forwardThe amount of heat per second conducted from the blood capillaries beneath the skin to the surface is 260 J/s. The energy is transferred a distance of 1.5 x 103 m through a body whose surface area is 1.4 m². Assuming that the thermal conductivity is that of body fat, determine the temperature difference between the capillaries and the surface of the skin.arrow_forward
- A sphere of surface area 1.25 m² and emissivity 1.0 is at a temperature of 100°C. At what rate does it radiate heat into empty space? (o = 5.67 × 10-8 W/m2.K4) O 3.7 W O 0.71 mW O 1.4 kW O 7.1 W O 9.9 mWarrow_forwardA closed box is filled with dry ice at a temperature of -83.9 °C, while the outside temperature is 26.9 °C. The box is cubical, measuring 0.350 m on a side, and the thickness of the walls is 3.80 x 10-2 m. In one day, 3.35 x 106 J of heat is conducted through the six walls. Find the thermal conductivity of the material from which the box is made. Number 30251.3 Unitsarrow_forwardA piece of iron block moves across a rough horizontal surface before coming to rest. The mass of the block is 2.8 kg, and its initial speed is 2.2 m/s. How much does the block's temperature increase, if it absorbs 78% of its initial kinetic energy as internal energy? The specific heat of iron is 452 J/(kg · °C). 0.004 X °Carrow_forward
- A closed box is filled with dry ice at a temperature of -86.0 °C, while the outside temperature is 21.0 °C. The box is cubical, measuring 0.394 m on a side, and the thickness of the walls is 4.49 × 102 m. In one day, 3.76 × 106 J of heat is conducted through the six walls. Find the thermal conductivity of the material from which the box is made. Number Unitsarrow_forwardA thermally isolated container has 479 grams of water in it and a 376 gram plastic block. The water had an initial temperature of 352 K. The plastic had an initial temperature of 300 K. The plastic and water reach an equilibrium temperature of 342 K. Water has a specific heat of 4.182 J/(gram K). What is the specific heat of the plastic block in J/(gram K)?arrow_forwardA copper block is removed from a 300 ∘C oven and dropped into 1.30 kg of water at 22.0 ∘C. The water quickly reaches 32.0 ∘C and then remains at that temperature. What is the mass of the copper block? The specific heats of copper and water are 385 J/(kg⋅K) and 4190 J/(kg⋅K) respectively. Express your answer to three significant figures and include the appropriate units.arrow_forward
- How much energy is required to heat 8.6kg of ice from -20℃ to steam at 120C ? The specific heat capacity of ice is 2.097 J/kg C The latent heat of fusion of ice is: Lf = 3.33 × 105J/kg The specific heat capacity of water is 4186J/kg C The latent heat of vaporization of water is: Lv = 22.6 × 105J/kg The specific heat capacity of steam is 1996 J/kg C DO NOT include units. DO NOT write in scientific notation. Do not include any "spaces" in your answer. 1. The heat energy needed to raise the temperature of ice to 0 degrees Celcius (in Joules) = 2. The heat energy needed to convert ice to water at 0 degrees Celcius (in Joules) = 3. The heat energy needed to raise the temperature of water from 0 degrees to 100 degrees Celcius (in Joules)= 4. The heat energy needed to convert water to steam at 100 degrees Celcius (in Joules) = 5. The heat energy needed to raise the temperature of steam from 100 to 120 degrees Celcius (in Joules) = 6. Using the answers above, the total energy to convert ice at…arrow_forwardThe temperature of a aluminum bar rises by 10.0°C when it absorbs 4.73 kJ of energy by heat. The mass of the bar is 525 g. Determine the specific heat of aluminum from these data.arrow_forwardHow much thermal energy (in J) is required to boil 2.45 kg of water at 100.0°C into steam at 135.0°C? The latent heat of vaporization of water is 2.26 ✕ 106 J/kg and the specific heat of steam is 2010 J kg · °C . HINT Jarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON