
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
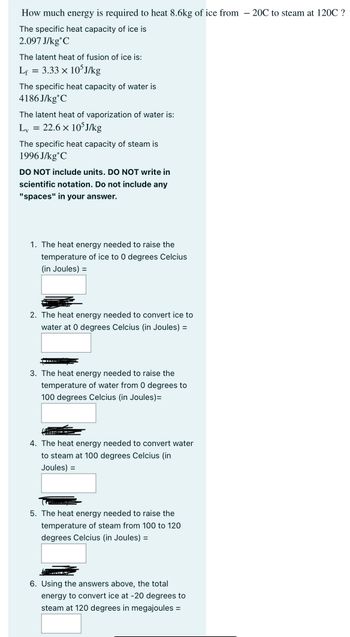
Transcribed Image Text:How much energy is required to heat 8.6kg of ice from -20℃ to steam at 120C ?
The specific heat capacity of ice is
2.097 J/kg C
The latent heat of fusion of ice is:
Lf
=
3.33 × 105J/kg
The specific heat capacity of water is
4186J/kg C
The latent heat of vaporization of water is:
Lv
=
22.6 × 105J/kg
The specific heat capacity of steam is
1996 J/kg C
DO NOT include units. DO NOT write in
scientific notation. Do not include any
"spaces" in your answer.
1. The heat energy needed to raise the
temperature of ice to 0 degrees Celcius
(in Joules) =
2. The heat energy needed to convert ice to
water at 0 degrees Celcius (in Joules) =
3. The heat energy needed to raise the
temperature of water from 0 degrees to
100 degrees Celcius (in Joules)=
4. The heat energy needed to convert water
to steam at 100 degrees Celcius (in
Joules) =
5. The heat energy needed to raise the
temperature of steam from 100 to 120
degrees Celcius (in Joules) =
6. Using the answers above, the total
energy to convert ice at -20 degrees to
steam at 120 degrees in megajoules
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- An ice tray is removed from a freezer, where the ice was at a temperature of -11°C, and left on a countertop. If the mass of the ice is 0.29 kg, how much heat must be added in k to turn all the ice into room temperature water (that is, liquid water at 20°C)? The specific heat of water is 4.2 kgC kJ the heat of fusion of water is 335 kg kJ and the specific heat of ice is 2.1 kg°C* 133.69 This is a change of phase question. There are 3 cases we have to consider: 1. The heat required to increase the temperature of the ice, Q1 2. The heat required to turn the ice into a liquid (a phase change), Q2 3. The heat required to raise the temperature of the now liquid water, Q3 For Q1, you will use the equation Q = mcATusing the c=2.1 kJ/(kg*C). Keep in mind that the hparrow_forwardHow much heat energy in unit calorie is given up when 2 0.0 g of steam at 100.0 oC is condensed and cooled to 20.0 oC?arrow_forwardA 50 g ice cube at 00C is heated until 45 g has become water at 1000C and 5 g has become steam at 1000C. Determine the amount of heat that can be added to accomplish this? [ Cwater = 4186Jkg-1k-1, Lv = 2.26 * 10⁶ Jkg-1, Lf=3.33 * 105Jkg-1arrow_forward
- An unknown piece of metal is heated to 98.0°C and dropped into a coffee cup calorimeter containing 250.0 g of water. The water in the calorimeter increases from 2.15°C to 23.0°C. the specific heat of water is 4.18 J/g°C. calculate the energey of the metal.arrow_forwardWhat quantity of joules of energy are needed to transform 11 kg of ice at 0.00°C to vapor at 155°C? Specific heat of water is cwater = 4186 J/kg C° Specific heat of steam is csteam = 2010 J/kg C° Latent heat of fusion of water is Lfusion = 3.33 X 105 J/kg Latent heat of vaporization of water is Lvaporization = 2.26 X 106 J/kg Boiling point of water = 100° Carrow_forwardAn aluminum rod is heated to 245 ºC and placed in a coffee cup calorimeter containing 250.0 g of water at 22.0 ºC. After the system has reached thermal equilibrium, the temperature of the water is found to be 49.0 ºC. What was the mass of the rod? Assume no heat is lost to the calorimeter.Cs(Al) = 0.921 J g-1 ºC-1Cs(H2O) = 4.18 J g-1 ºC-1arrow_forward
- A 85 g iron bar (specific heat =450J/kg C) at 100 °C is placed into 230 g of water at 15 "C. What will be the final temperature of the iron bar as it cools in the water? Your Answer: Answerarrow_forwardIce at 0 degree C with a mass of 200 g is tossed into a pot of boiling water. The boiling water is at 100 degree C and has a mass of 1 kg. What is the final temperature of the water after the ice melts? (Lf = 3.33 x 10^5 J/kg, Cwater 4186 J/kg degreeC)arrow_forward1) A Sample Of Water That Weigh 25g Absorbs 880J Of Energy. The Water Is Initially At 38.0 'C. What Is The New Temperature Of The Water In Degrees Fahrenheit? 2) A Sample Of Metal, Heated To 110'C, Is Placed Into A Calorimeter Containing 140g Of Water At 15.0'C. The Final Temperature Of The Metal And Water Is 28.0'C. What Is The Mass Of The Metal (m Metal)? Water Q= Cs= 4.184 M = Tfinal F Tinitial = AT = Metal Q= Cs= 0.628 M= Tfinal Tinitial = AT =arrow_forward
- A 450 mL cup of coffee is too hot to drink at 91C, so 100.0 mL of cold water at 6.5 C is added to cool it off. What is the final temperature of the drink?arrow_forwardTo treat a burn on his hand, a person decides to place an ice cube on the burned skin. The mass of the ice cube is 16.8 g, and its initial temperature is -10.7 °C. The water resulting from the melted ice reaches the temperature of his skin, 29.4 'C. How much heat is absorbed by the ice cube and resulting water? Assume that all of the water remains in the hand. Constants for water can be found in this table.arrow_forwardHow much heat energy must be removed from 0.10 kg of oxygen with a temperature of 22 °C in order for the oxygen to liquefy at -183 °C? (Co = 913 J/kg°C, Ly = 2.13 x 105 J/kg and oxygen liquefies as -183 °C) O 5 x 10^4 J O 9 x 10^4 J O 1 x 10^4 J O 4 x 10^4Jarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON