
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN: 9781305580343
Author: Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
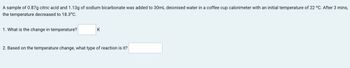
Transcribed Image Text:A sample of 0.87g citric acid and 1.13g of sodium bicarbonate was added to 30mL deionised water in a coffee cup calorimeter with an initial temperature of 22 °C. After 3 mins,
the temperature decreased to 18.3°C.
1. What is the change in temperature?
K
2. Based on the temperature change, what type of reaction is it?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- A 21.3-mL sample of 0.977 M NaOH is mixed with 29.5 mL of 0.918 M HCl in a coffee-cup calorimeter (see Section 6.6 of your text for a description of a coffee-cup calorimeter). The enthalpy of the reaction, written with the lowest whole-number coefficients, is 55.8 kJ. Both solutions are at 19.6C prior to mixing and reacting. What is the final temperature of the reaction mixture? When solving this problem, assume that no heat is lost from the calorimeter to the surroundings, the density of all solutions is 1.00 g/mL, the specific heat of all solutions is the same as that of water, and volumes are additive.arrow_forwardA student performing a calorimetry experiment combined 100.0 ml. of 0.50 M HCI and 100.0 ml. of 0.50 M NaOH in a StyrofoamTM cup calorimeter. Both solutions were initially at 20.0 C, but when the two were mixed, the temperature rose to 23.2 C (a) Suppose the experiment is repeated in the same calorimeter but this time using 200 mL of 0.50 M HCl and 200.0 ml of 0.50 M NaOH. WIII the AT observed be greater than, less than, or equal to that in the first experiment, and why? (b) Suppose that the experiment is repeated once again in the same calorimeter, this time using 100 mL of 1.00 M HCI and 100.0 ml. of 1.00 M NaOH. Will the T observed be greater than, less than, or equal to that in the first experiment, and why?arrow_forwardThe decomposition of ozone, O3, to oxygen, O2, is an exothermic reaction. What is the sign of q? If you were to touch a flask in which ozone is decomposing to oxygen, would you expect the flask to feel warm or cool?arrow_forward
- A 50-mL solution of a dilute AgNO3 solution is added to 100 mL of a base solution in a coffee-cup calorimeter. As Ag2O(s) precipitates, the temperature of the solution increases from 23.78 C to 25.19 C. Assuming that the mixture has the same specific heat as water and a mass of 150 g, calculate the heat q. Is the precipitation reaction exothermic or endothermic?arrow_forwardConsider the reaction 2HCl(aq)+Ba(OH)2(aq)BaCl2(aq)+2H2O(l)H=118KJ Calculate the heat when 100.0 rnL of 0.500 M HCl is mixed with 300.0 mL of 0.100 M Ba(OH)2 Assuming that the temperature of both solutions was initially 25.0C and that the final mixture has a mass of 400.0 g and a specific heat capacity of 4.18 J/C g, calculate the final temperature of the mixture.arrow_forwardAn industrial process for manufacturing sulfuric acid, H2SO4, uses hydrogen sulfide, H2S, from the purification of natural gas. In the first step of this process, the hydrogen sulfide is burned to obtain sulfur dioxide, SO2. 2H2S(g)+3O2(g)2H2O(l)+2SO2(g);H=1124kJ The density of sulfur dioxide at 25C and 1.00 atm is 2.62 g/L, and the molar heat capacity is 30.2 J/(mol C). (a) How much heat would be evolved in producing 1.00 L of SO2 at 25C and 1.00 atm? (b) Suppose heat from this reaction is used to heat 1.00 L of the SO2 from 25C to 500C for its use in the next step of the process. What percentage of the heat evolved is required for this?arrow_forward
- The thermochemical equation for the burning of methane, the main component of natural gas, is CH4(g)+2O2(g)CO2(g)+2H2O(l)H=890kJ (a) Is this reaction endothermic or exothermic? (b) What quantities of reactants and products are assumed if H = 890 kJ? (c) What is the enthalpy change when 1.00 g methane burns in an excess of oxygen?arrow_forwardIn a coffee-cup calorimeter, 150.0 mL of 0.50 M HCI is added to 50.0 mL of 1.00 M NaOH to make 200.0 g solution at an initial temperature of 48.2C. If the enthalpy of neutralization for the reaction between a strong acid and a strong base is 56 kJ/mol, calculate the final temperature of the calorimeter contents. Assume the specific heat capacity of the solution is 4.184 J/g C and assume no heat Joss to the surroundings.arrow_forwardWhen solid iron burns in oxygen gas (at constant pressure) to produce Fe2O3(s), 1651 kJ of heat is released for every 4 mol of iron burned. How much heat is released when 10.3 g Fe2O3(s) is produced (at constant pressure)? What additional information would you need to calculate the heat released to produce this much Fe2O3(s) if you burned iron in ozone gas, O3(g), instead of O2(g)?arrow_forward
- 9.83 A student performing a calorimetry experiment combined 100.0 mL of 0.50 M HCl and 100.0 mL of 0.50 M NaOH in a coffee cup calorimeter. Both solutions were initially at 20.0°C, but when the two were mixed, the temperature rose to 23.2°C. (a) Suppose the experiment is repeated in the same calorimeter but this time using 200 mL of 0.50 M HCl and 200.0 mL of 0.50 M NaOH. Will the T observed he greater than, less than, or equal to that in the first experiment, and why? (b) Suppose that the experiment is repeated once again in the same calorimeter, this time using 100 mL of 1.00 M HCl and 100.0 mL of 1.00 M NaOH. Will the T observed he greater than, less than, or equal to that in the first experiment, and why?arrow_forwardA sample of sucrose, C12H22O11, is contaminated by sodium chloride. When the contaminated sample is burned in a bomb calorimeter, sodium chloride does not burn. What is the percentage of sucrose in the sample if a temperature increase of 1.67C is observed when 3.000 g of the sample are burned in the calorimeter? Sucrose gives off 5.64103kJ/mol when burned. The heat capacity of the calorimeter and water is 22.51 kJ/C.arrow_forwardIn a bomb calorimeter, the reaction vessel is surrounded by water that must be added for each experiment. Since the amount of water is not constant from experiment to experiment, the mass of water must be measured in each case. The heat capacity of the calorimeter is broken down into two parts: the water and the calorimeter components. If a calorimeter contains 1.00 kg water and has a total heat capacity of 10.84 kJ/C, what is the heat capacity of the calorimeter components?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781285199023
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning