
Chemistry for Today: General, Organic, and Biochemistry
9th Edition
ISBN: 9781305960060
Author: Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
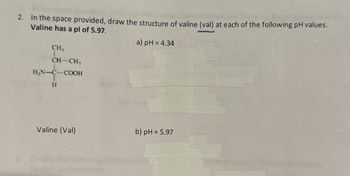
Transcribed Image Text:2. In the space provided, draw the structure of valine (val) at each of the following pH values.
Valine has a pl of 5.97.
CH₁
CH-CH,
H₂N-C-COOH
H
a) pH = 4.34
Valine (Val)
b) pH = 5.97
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 3 images

Knowledge Booster
Similar questions
- What special role does the amino acid cysteine have in the peptides vasopressin and oxytocin?arrow_forwardDraw a segment of the backbone of a protein that is long enough for three peptide linkages to be present.arrow_forwardFor the tripeptide AlaValGly which amino acid residues, if any, a. are hydrophilic b. are hydrophobic c. possess nonpolar R groups d. participate in two amide linkagesarrow_forward
- What characteristics indicate that amino acids exist as zwitterions?arrow_forwardFor the tripeptide SerArgIle which amino acid residues a. are hydrophilic b. are hydrophobic c. possess polar neutral R groups d. participate in two amide linkagesarrow_forwardConsider the tripeptide leucylvalyltryptophan. a. Specify its structure using three-letter symbols for the amino acids. b. How many peptide bonds are present within the peptide? c. Which of the amino acid residues has the largest R group? d. Which of the amino acid residues, if any, has a basic side chain?arrow_forward
- In the space provided, draw the structure of valine (val) at each of the following pH values. Valine has a pl of 5.97. a) pH = 4.34 CH3 CH-CH, H2N-C-COOH Valine (Val) b) pH = 5.97 %3Darrow_forwardDraw the sequential transition of glutamic acid as it passes from its fully protonated form to its fully deprotonated form as the pH rises. If the pH of an amino acid solution is lowered by adding an acid, like , the group of glutamic acid accepts the proton, acid, to form a positive ion.arrow_forwardWhat type of amino acid has a side chain that contains –COO–? polar neutral nonpolar acidic basic conjugate basicarrow_forward
- Please don't provide handwritten solution ..arrow_forward2. Classify the following amino acids as nonpolar, polar basic, polar acidic, or polar neutral. (a) (b) (c) (d) H₂N-CH-COH CH-OH I CH3 || H₂N-CH-C-OH I CH₂ C=O 1 OH H₂N-CH-C-OH CH₂ T CH-CH3 CH₂ H₂N-CH-C-OH ī CH₂ OH 229arrow_forwardWrite the conjugate acid of CH3CH3O-arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning