
Fundamentals Of Analytical Chemistry
9th Edition
ISBN: 9781285640686
Author: Skoog
Publisher: Cengage
expand_more
expand_more
format_list_bulleted
Question
help please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all working
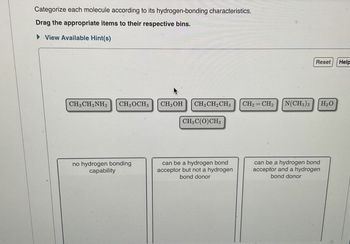
Transcribed Image Text:Categorize each molecule according to its hydrogen-bonding characteristics.
Drag the appropriate items to their respective bins.
View Available Hint(s)
Reset
Help
CH3CH2NH2 CH3OCH3
CH3OH
CH3CH2CH3
CH2=CH2
N(CH3)3
H₂O
CH3C(O)CH3
no hydrogen bonding
capability
can be a hydrogen bond
acceptor but not a hydrogen
bond donor
can be a hydrogen bond
acceptor and a hydrogen
bond donor
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- nic Chem OWLv2 | Online teaching and le X WhatsApp akeAssignment/takeCovalentActivity.do?locator-assignment-take [References) + Carbon-hydrogen bonds exhibit a range of different chemical reactivity that depends on molecular structure. Classify the C-H bonds at the carbons labeled a-c in the structure below. Possible classifications are: primary, secondary, & tertiary or none if there are no hydrogens at the labeled carbon. C-H bond(s) at al C-H bond(s) at b C-H bond(s) at c Submit Answer Retry Entire Group 9 more group attempts remaining F4 % F5 Cengage Learning | Cengage Technical Support * Previous Email Instructor PrtScn Home End PgUp PgDn F6 F7 FB F9 F10 F11 F12 * 00 ( 9 + Save are วarrow_forwardCarbon-hydrogen bonds exhibit a range of different chemical reactivity that depends on molecular structure. Classify the C-H bonds at the carbons labeled a-c in the structure below.arrow_forwardFor the molecule shown below, please answer the following questions. Enter only numerals (e.g. 1") rather than words (e.g. "one"). NH3 HO OH NH2 OH OH O How many hydrogen atoms are there? A How many p-orbitals are involved in n bonds in this molecule? How many Inn iarrow_forward
- The bond dissociation energy to break 2 C-C bond(s) in 1 mole of glycerol (HOCH2CH(OH)CH2OH, see image below) molecules is H -C H- Single Bond H C N H 432 411 346 N 386 305 167 459 358 201 142 C=C 602 C=0 799 Multiple C=C 835 C=0 1072 Bonds 615 494 C=N O=0 CEN 887 N=N 942 "Al vales in kaarrow_forwardI need help woth this question pleasearrow_forward19. Based on molecular mass and dipole moment of the five compounds in the table below, which one should have the highest boiling point? Dipole Moment (D) Substance Molecular Mass (amu) Propane, CH3CH2CH3 Dimethyl ether, CH3OCH3 Methylchloride, CH3C1 Acetaldehyde, CH;CHO Acetonitrile, CH3CN 44 0.1 46 1.3 50 1.9 44 2.7 41 3.9 B) CH;CN E) CH3CH2CH3 C) CH3CHO A) CH3OCH3 D) CH;CIarrow_forward
- Please draw the two intermediates A and Cand then predict the products from the given reaction B and D, showing all potential products (including stereoisomers if they exist). Short3.png + (48KB)arrow_forwardIn a sample of bonds in the position. (Note: Remember that iodine () is larger and more likely to contribute to steric strain in the molecule.) iodocyclohexane (CH), one would expect to find most of the C-I O equatorial O trigonal pyramidal O tetrahedral Oplanar O axial Question 7 The molecule aniline is pictured here. Which of the following words could be used to describe an essential feature of aniline to someone who is familiar with chemistry, but not with this molecule? O aromatic O heterocycle O non-cyclic fused-ring system O alkane NH₂arrow_forwardWhich of the following statements are TRUE for covalent pi bonds? (Choose as many as apply. Hint: There are 4 correct answers!) Pi bonds arise from head-on overlap of two orbitals. Pi bonds arise from sideways overlap of two orbitals. The electron density in a pi bond is found above and below the axis between the two bonded nuclei. The electron density in a pi bond is found in between the two bonded nuclei. A pi bond can exist independently; not every pi bond is accompanied by an associated o bond. Pi bonds always exist in conjunction with an associated o bond; they can never exist independently. The pi bonds in a molecule determine the shape of the molecule. There can be more than one pi bond between two nuclei. Every covalent bond contains at least one pi bond.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning