
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
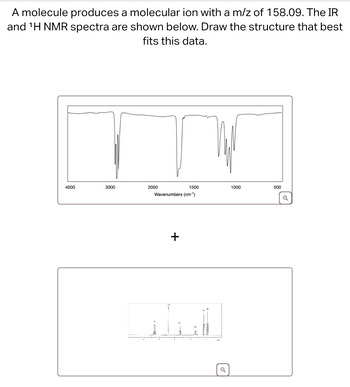
Transcribed Image Text:A molecule produces a molecular ion with a m/z of 158.09. The IR
and 'H NMR spectra are shown below. Draw the structure that best
fits this data.
4000
3000
2000
1500
1000
500
Wavenumbers (cm-1)
Q
+
Q
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 4 images

Knowledge Booster
Similar questions
- When the 1HNMR spectrum of an alcohol is run in dimethylsulfoxide (DMSO) solvent rather than in chloroform, exchange of the Ο-H proton is slow and spin-spin splitting is seen between the Ο-H proton and C-H protons on the adjacent carbon. What spin multiplicities would you expect for the hydroxyl protons in the following alcohols? (a) 2-Methyl-2-propanol (b) Cyclohexanol (c) Ethanol (d) 2-Propanol (e) Cholesterol (f) 1-Methylcyclohexanolarrow_forward3-Bromo-1-phenyl-1-propene shows a complex NMR spectrum in which the vinylic proton at C2 is coupled with both the C1 vinylic proton (J = 16 Hz) and the C3 methylene protons (J = 8 Hz). Draw a tree diagram for the C2 proton signal, and account for the fact that a five-line multiplet is observed.arrow_forwardThe mass spectrum and 13C NMR spectrum of a hydrocarbon are shown. Propose a structure for this hydrocarbon, and explain the spectral data.arrow_forward
- A'H NMR spectrum is shown for a molecule with the molecular formula of CeH9Br. Draw the structure that best fits this data.arrow_forwardBased on this 'H NMR spectrum, choose the compound that is the most likely to match the integration, shift, and multiplicity. C B A III = D IV H.CO I ||arrow_forwardHow many peaks would the 1H NMR spectrum of the following molecule have?arrow_forward
- A 'H NMR spectrum is shown for a molecule with the molecula formula of C9H10O2. Draw the structure that best fits this dataarrow_forwardThe IR and 13C NMR spectra are shown for a molecule with the molecular formula of C6H10. Draw the structure that best fits this data.arrow_forwardA molecule produces a molecular ion with a m/z of 86 and a base peak with a m/z of 44. The IR and ¹H NMR spectra are shown below. Draw the structure that best fits this data. 4000 3000 11 2000 14 L 10 7 1500 Warumbesc + 1000 2H 21 24 1 500 DH apny Q Qarrow_forward
- A 13C NMR spectrum is shown for a molecule with the molecular formula of CsH1002. Draw the structure that best fits this data. 180 160 140 120 100 PPM 80 60 40 20 O Qarrow_forwardA molecule produces two molecular ions with a m/z of 152 and 154 and a base peak with a m/z of 73. The IR and ¹H NMR spectra are shown below. Draw the structure that best fits this data. 4000 1H 11 10 3000 9 8 2000 7 1500 Wavenumbers (cm) + 6 5 2H 1000 2H 2 1 500 ppm of Q Qarrow_forwardWhich compound is correct for the given NMR spectrum? |(3) (2) (2) 2 1 PPM CN 1) 2) 3) Structure 1 O Structure 2 Structure 3arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning