
Fundamentals Of Analytical Chemistry
9th Edition
ISBN: 9781285640686
Author: Skoog
Publisher: Cengage
expand_more
expand_more
format_list_bulleted
Question
Please correct answer and don't use hand rating
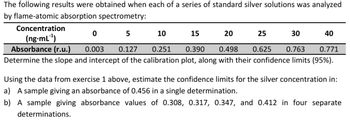
Transcribed Image Text:The following results were obtained when each of a series of standard silver solutions was analyzed
by flame-atomic absorption spectrometry:
Concentration
(ng-mL¹)
Absorbance (r.u.)
0
5
10
15
20
25
30
40
0.003
0.127
0.251
0.390
0.498
0.625
0.763
0.771
Determine the slope and intercept of the calibration plot, along with their confidence limits (95%).
Using the data from exercise 1 above, estimate the confidence limits for the silver concentration in:
a) A sample giving an absorbance of 0.456 in a single determination.
b) A sample giving absorbance values of 0.308, 0.317, 0.347, and 0.412 in four separate
determinations.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- Mass spectrometry is an extremely versatile detection system for GC. However, interfacing an HPLC system to a mass spectrometer is a much more difficult task. Describe the major reasons why it is more difficult to combine HPLC with mass spectrometry than it is to combine GC with mass spectrometry.arrow_forwardA solution is prepared by diluting 2.79 mL of the blue dye stock solution to 25.00 mL. The measured absorbance for the prepared solution is: Blue dye stock solution = 0.293 M Absorbance at 630 nm = 0.00265 Calibration curve y = 0.0833x A.) What is the theoretical molar concentration? B.) What is the experimental molar concentration? C.) What is the percent error?arrow_forward0.1040 g of a solid sample containing copper was dissolved by acid and transferred to a 250.00 mL volumetric flask and volume was made up with distilled water. The solution was then diluted by a factor of 20. The final solution was analyzed by atomic absorption spectrophotometry and the concentration was found be 3.109 ppm, What is the weight percentage of copper in the original solid sample? Keep four significant figures in your final answer. Do not try to include the "%" in your answer. e.g. if your final answer is 10.01%, only write 10.01 in the box.arrow_forward
- Please answer all required.arrow_forwardAn unknown sample of Yellow 6 solution has the absorbance equal to 0.96. The slope of the Beer's law calibration plot constructed using a 3.8E-5M stock solution of Yellow 6 is 0.0117 (%) 1. What is the molar concentration of the unknown sample (in mole L-¹)? Please give your answer to 3 significant figures (1 additional SF is used to minimize rounding errors). M Hintarrow_forward0.1120 g of a solid sample containing copper was dissolved by acid and transferred to a 250.00 mL volumetric flask and volume was made up with distilled water. The solution was then diluted by a factor of 25. The final solution was analyzed by atomic absorption spectrophotometry and the concentration was found be 2.982 ppm, What is the weight percentage of copper in the original solid sample? Keep four significant figures in your final answer.arrow_forward
- Flame Atomic Absorption Spectroscopy (FAAS) method is selected due to its wide applicability for metal ion analysis in drinking water samples. Provide appropriate discussion to determine the concentration of selected heavy metals (Cu and Cr) in the unknown tap water sample.arrow_forwardNonearrow_forwardCc.58.arrow_forward
- Prepare a calibration curve to determine the Pb content in a well water sample using atomic absorption spectrophotometry; the following data were recorded: % T 100 75.2 56.6 42.5 31.9 24.6 13.8 ppm Pb 0 0.2 0.4 0.6 0.8 1.0 1.4 A 50 mL aliquot of well water is taken and diluted twice, the% T reading is taken and a value of 40.6% is recorded. Calculate the ppm of Pb in the well water.arrow_forwardI need the answer as soon as possiblearrow_forwardWhat is the extinction coefficient for the following absorbances? 1. 3.000 2. 2.022 3.1.2025 4. 0.824 Would the units be in µmoles/mL)^-1 cm^-1arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning