
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
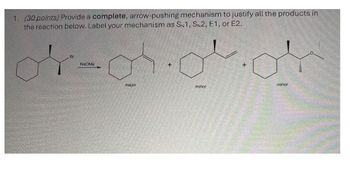
Transcribed Image Text:1. (30 points) Provide a complete, arrow-pushing mechanism to justify all the products in
the reaction below. Label your mechanism as SN1, SN2, E1, or E2.
Br
NaOMe
major
+
minor
+
minor
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 7 images

Knowledge Booster
Similar questions
- (4d) and PhMgBr, followed by reaction with the acid. Please draw that mechanism here! Even though benzoic acid is reformed as the product, a reaction does take place between benzoic acid 1. PhMgBr HO, 2. H30*arrow_forward3. (15 points) Complete the reaction scheme below. Show all reagents and intermediates. No reaction is a possible answer. H20 a) H20 LIAIH4 b) H3O*arrow_forward. Use to curved arrow notation, propose a mechanism for the following reaction and state whether it is either SN1, E1, SN2, or E2. Give the IUPAC names of all organic reactants and products.arrow_forward
- 1. (10 points) Complete the reaction scheme below. Show all reagents and intermediates. No reaction is a possible answer. Do not neglect stereochemistry. NaCN DIBAL Br A "OCH3 (sodium cyanide)arrow_forwardPredict the major product of the given reaction and then draw a reasonable mechanism for the product formation using appropriate curved arrow notation. Part 1: OH H2SO4 (aq) view structure Part 2 out of 4 H,0 H,SO4 HSO, HO finish structure . draw structure . H;O*arrow_forward(3) Mechanism. Provide the mechanism for the following reaction. HO OH Он |-0 HO Он NaOH, Н2О но HO Он HO Онarrow_forward
- 4. (10 points) Complete the reaction scheme below. Show all reagents and intermediates. No reaction is a possible answer. LDA lodomethane CH3 H Harrow_forwardiii) 10. For each of the following reactions, provide the structure of the major product and circle the predominant mechanism. Indicate the stereochemistry where necessary. VII) E1 E2 SN1 SN2 E1 E2 SN1 SN2 NaSH DMF H* CH3OH Br (CH3)3COK (CH3)3COH OH iv) HBr viil) E1 E2 E1 E2 SN1 SN2 SN1 SN2arrow_forwardDraw the major organic product generated in the reaction below. Pay particular attention to regio- and stereochemical detail. CH₂CH₂CH3 НО. Provide a detailed, step-by-step mechanism for the reaction shown below. 1.03 2. (CH₂)₂S Br₂arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning