
World of Chemistry, 3rd edition
3rd Edition
ISBN: 9781133109655
Author: Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher: Brooks / Cole / Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Hi can you show me the
Thank you very much.
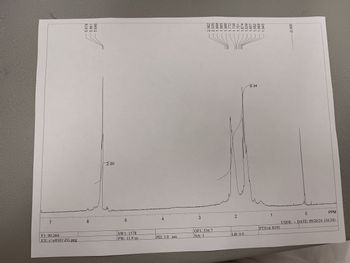
Transcribed Image Text:2.00
7
6
5
F1: 90.264
EX: c:\eft\H1\ZG.ppg
4
3
2
2.062
2.026
1.998
1.985
1.966
1.772
1.738
1.721
1.674
1.639
1.607
1.582
1.569
1.543
SW1: 1578
PW: 11.9 us
PD: 3.0 sec
OF1: 536.7
NA: 1
PTS1d: 8192
LB: 0.0
1
9.34
0
PPM
USER: --DATE: 09/20/24 (16:34)
-0.000
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- nHMAC%3Def41415d9158412623399163083b2ede#10001 Spr. 2021 8 Gu HI, Leira v Sign Out Item 4 I Review | Constants | Periodic Table Alexandra decides to climb Mt. Krumpett, which is 5000 m high. She determines that this will require a total of 2250 kcal of energy for the trip. For her food supply, she decides to take nutrition bars. The label states that each bar contains 50 g of carbohydrates, 10 g of fat, and 40 g of protein. Part A How many nutrition bars should Alexandra pack? Express the number of bars numerically. > View Available Hint(s) ν ΑΣφ bars Submit Previous Answers 0V 12:33arrow_forwarda .ll Asiacel| 10 N 141 N O A box with a volume (V=0.05 m³) lies at the bottom of a lake whose water has a density of (1*10³ kg/m³). How much force is required to lift the box, .if the mass of the box is (1000 kg) 9319.5 N O 9313.9 N O 9391.5 N O 9315.9 N O A future rectangular ship filled with oil. If the dimensions of the ship are (250 m) long, (80 m) wide, and (80 m) high. Determine how far the bottom of the ship is below the sea level? (Consider the total mass of the ship with the oil is (10.2*108 kg), and the .sea density is (1024 kg/m³))arrow_forward2. The following eight structures are all isomers of C6H14O. Each structure corresponds to one of the predicted ¹H NMR spectra pictured on the next two pages of this document. The integral values for each peak are next to that peak. Indicate which structure matches each spectrum by drawing the structures in the boxes along the right side of the pages. Use the letter codes provided to label the chemically equivalent hydrogens on the structures. One example is provided. Xor A 2H 1H <- A 4H -3₂₂ OH B 2H tot 2 PPM PPM -N 2 PPM N- -~ B 12H DE C 2H 2H 1H B 4H 6H ха по мон F 6H B B B warrow_forward
- a .ull Asiacel| 10 N 141 N O A box with a volume (V=0.05 m³) lies at the bottom of a lake whose water has a density of (1*10³ kg/m³). How much force is required to lift the box, .if the mass of the box is (1000 kg) 9319.5 N O 9313.9 N O 9391.5 N O 9315.9 N O A future rectangular ship filled with oil. If the dimensions of the ship are (250 m) long, (80 m) wide, and (80 m) high. Determine how far the bottom of the ship is below the sea level? (Consider the total mass of the ship with the oil is (10.2*108 kg), and the .sea density is (1024 kg/m³))arrow_forward+ e Home ourse.html?courseld=15539865&HepID=676f177415469ec3dc78ff3c8 bf724d9#1 0001 Inbox-Outlook We... S Sugarpill Cosmetic... Shop | Origami Owl Wholesale Glass M... S The Corinthian Hou... zed Invitat... HW Provide Feedback P Pearson Contact Us Copyright 2019 Pearson Education Inc. All rights reserved. Terms of Use Privacy Policy | Permissions MacBook Air »)) DD DII O00 F12 F11 F10 F9 F8 F7 F4 F6 F5 F3 + $ & 3 0 9 4 7 8 6 5 } { P E U R Y D L K G F H T 2LOarrow_forward1s²25²2p°3s²3parrow_forward
- HCT E-Learning Portal ics 1 Engineering Pract y courses / Applied Science / Physics 1 Engineering Pra What is unit of coeffic of friction? a. No unit of b. Watt с. Кg d. Newton us page Announcements Tn Add Moodle toarrow_forwardP Cati x appl x Curr x w Revis x Cher x M Your x C Get x C Scor X 10, Che x C 3:08 x C 3:09 X Scor X G sn p x + app.101edu.co High Kick Through.. >> M Mathway | Algebra.. K https://www.netflix.. Log In to Canvas BROCKTON CharlieCard Web Pr... Watch Korean dra.. Question 15 of 15 How many grams of iron metal can be formed by the reaction of 2.14 g Al metal with excess Fe2O3, according to the thermite reaction: 2 Al(s) + Fe,O, Fe(s) + Al,O3(s) STARTING AMOUNT ADD FACTOR ANSWER RESET *( ) 26.98 4.43 6.022 x 102 55.85 102.0 4.05 6.33 2. 2.14 1. 159.7 4.78 x 1022 moi Al n Co. mal ALO. A ALO mal Co.0 mol Coarrow_forwardAssuming a large number of measurements so that s is a good estimate of σ , determine what confidence level was used for each of the following confidence intervals.arrow_forward
- 13.Calibration must be properly done to ensure that glass ware and equipment are in good working condition. Anybody can perform calibration of an equipment.a.Both statements are correctb.Both statements are incorrectc.The first statement is incorrect while the second statement is correctd.The first statement is correct while the second statement us incorrect14.Glass measures are preferred for measuring liquids becausea.All of theseb.It is easy to handlec.Of its coefficient of expansiond.Transparency of the glass can more accurately indicate volumearrow_forward... The prefix giga is used in the metric system has a value of 1016 A lo19 10+12arrow_forwardRun a simulation to determine if buying 23 boxes is unusualarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781285199023
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning