
Chemistry: Principles and Practice
3rd Edition
ISBN: 9780534420123
Author: Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:4
Part A
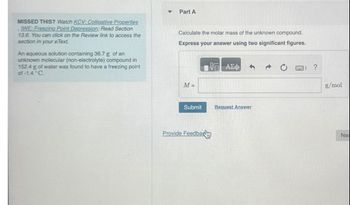
MISSED THIS? Watch KCV: Colligative Properties
IWE: Freezing Point Depression; Read Section
13.6. You can click on the Review link to access the
section in your eText.
An aqueous solution containing 36.7 g of an
unknown molecular (non-electrolyte) compound in
152.4 g of water was found to have a freezing point
of-1.4 °C.
Calculate the molar mass of the unknown compound.
Express your answer using two significant figures.
ΑΣΦ
M=
Submit
Request Answer
Provide Feedba
?
g/mol
Nex
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 4 images

Knowledge Booster
Similar questions
- A forensic chemist is given a white solid that is suspected of being pure cocaine (C17H21NO4, molar mass = 303.35 g/mol). She dissolves 1.22 0.01 g of the solid in 15.60 0.01 g benzene. The freezing point is lowered by 1.32 0.04C. a. What is the molar mass of the substance? Assuming that the percent uncertainty in the calculated molar mass is the same as the percent uncertainty in the temperature change, calculate the uncertainty in the molar mass. b. Could the chemist unequivocally state that the substance is cocaine? For example, is the uncertainty small enough to distinguish cocaine from codeine (C18H21NO3, molar mass = 299.36 g/mol)? c. Assuming that the absolute uncertainties in the measurements of temperature and mass remain unchanged, how could the chemist improve the precision of her results?arrow_forwardAn aqueous solution containing 10.0 g of starch per liter has an osmotic pressure of 3.8 mm Hg at 25 C. (a) What is the average molar mass of starch? (The result is an average because not all starch molecules are identical.) (b) What is the freezing point of the solution? Would it be easy to determine the molecular weight of starch by measuring the freezing point depression? (Assume that the molarity and molality are the same for this solution.)arrow_forwardThe freezing point of a 0.031-m solution of copper(II) sulfate in water is 0.075 C. (a) Calculate the vant Hoff factor, i, for this solution. (b) Would the vant Hoff factor be larger, smaller, or the same for a 0.050-m solution of this compound?arrow_forward
- A solution was prepared by dissolving 0.800 g of sulfur, Sg, in 100.0 g of acetic acid, HC2H3O2. Calculate the freezing point and boiling point of the solution.arrow_forwardHow do colloids differ from solutions with regard to dispersed particle size and homogeneity?arrow_forwardWhen KNO3 is dissolved in water, the resulting solution is significantly colder than the water was originally. (a) Is the dissolution of KNO3 an endothermic or an exothermic process? (b) What conclusions can you draw about the intermolecular attractions involved in the process? (c) Is the resulting solution an ideal solution?arrow_forward
- A gaseous solute dissolves in water. The solution process has H=15 kJ. Its solubility at 22C and 6.00 atm is 0.0300 M. Would you expect the solubility to be greater or less at (a) 22C and 1 atm? (a) 18C and 6 atm? (a) 15C and 10 atm? (a) 35C and 3 atm?arrow_forwardYou have read that adding a solute to a solvent can both increase the boiling point and decrease the freezing point. A friend of yours explains it to you like this: The solute and solvent can be like salt in water. The salt gets in the way of freezing in that it blocks the water molecules from joining together. The salt acts like a strong bond holding the water molecules together so that it is harder to boil. What do you say to your friend?arrow_forwardSodium chloride (NaCl) is commonly used to melt ice on roads during the winter. Calcium chloride (CaCl2) is sometimes used for this purpose too. Let us compare the effectiveness of equal masses of these two compounds in lowering the freezing point of water, by calculating the freezing point depression of solutions containing 200. g of each salt in 1.00 kg of water. (An advantage of CaCl2 is that it acts more quickly because it is hygroscopic, that is. it absorbs moisture from the air to give a solution and begin the process. A disadvantage is that this compound is more costly.)arrow_forward
- Calculate the freezing point of 525 g of water that contains 25.0 g of NaCl. Assume i, the vant Hoff factor, is 1.85 for NaCl.arrow_forwardSilver ion has an average concentration of 28 ppb (parts per billion) in U.S. water supplies. (a) What is the molality of the silver ion? (b) If you wanted 1.0 102 g of silver and could recover it chemically from water supplies, what volume of water in liters would you have to treat? (Assume the density of water is 1.0g/cm3.)arrow_forwarda. Calculate the freezing-point depression and osmotic pressure at 25C of an aqueous solution containing 1.0 g/L of a protein (molar mass = 9.0 104 g/mol) if the density of the solution is 1.0 g/cm3. b. Considering your answer to part a, which colligative property, freezing-point depression or osmotic pressure, would be better used to determine the molar masses of large molecules? Explain.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning