
Chemistry: Principles and Reactions
8th Edition
ISBN: 9781305079373
Author: William L. Masterton, Cecile N. Hurley
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
help please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all working!
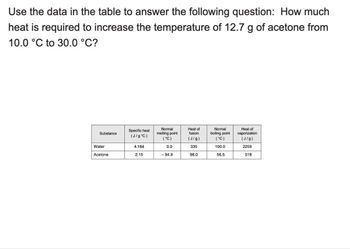
Transcribed Image Text:Use the data in the table to answer the following question: How much
heat is required to increase the temperature of 12.7 g of acetone from
10.0 °C to 30.0 °C?
Substance
Specific heat
(J/g°C)
Normal
melting point
(°C)
Heat of
fusion
(J/g)
Normal
boiling point
(°C)
Heat of
vaporization
(J/g)
Water
4.184
0.0
335
100.0
2259
Acetone
2.15
- 94.9
98.0
56.5
518
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Are changes in state physical or chemical changes? Explain. What type of forces must be overcome to melt or vaporize a substance (are these forces intramolecular or intermolecular)? Define the molar heat of fusion and molar heat of vaporization. Why is the molar heat of vaporization of water so much larger than its molar heat of fusion? Why does the boiling point of a liquid vary with altitude?arrow_forwardThe enthalpy of vaporization of water is larger than its enthalpy of fusion. Explain why.arrow_forwardThe enthalpy change when 1 mol methane (CH4) is burned is 890 kJ. It takes 44.0 kJ to vaporize 1 mol water. What mass of methane must be burned to provide the heat needed to vaporize 1.00 g water?arrow_forward
- Equal masses of liquid A, initially at 100C, and liquid B, initially at 50C, are combined in an insulated container. The final temperature of the mixture is 80C. All the heat flow occurs between the two liquids. The two liquids do not react with each other. Is the specific heat of liquid A larger than, equal to, or smaller than the specific heat of liquid B?arrow_forwardWhy does sweating cool the human body?arrow_forwardA quantity of ice at 0C is added to 64.3 g of water in a glass at 55C. After the ice melted, the temperature of the water in the glass was 15C. How much ice was added? The heat of fusion of water is 6.01 kJ/mol and the specific heat is 4.18 J/(g C).arrow_forward
- If you want to convert 56.0 g ice (at 0 °C) to water at 75.0 °C, calculate how many grams of propane, C3H8, you would have to bum to supply the energy to melt the ice and then warm it to the final temperature (at 1 bar).arrow_forwardWill a closed container of water at 70 C or an open container of water at the same temperature cool faster on a cold winter day? Explain why.arrow_forwardDefine the joule in terms of SI base units.arrow_forward
- If 14.5 kJ of heat were added to 485 g of liquid water, how much would its temperature increase?arrow_forward9.46 The heat of fusion of pure silicon is 43.4 kJ/mol. How much energy would be needed to melt a 5.24-g sample of silicon at its melting point of 1693 K?arrow_forwardFollow the step-wise process outlined in Problem 31 to calculate the amount of heat involved in condensing 100.00 g of benzene gas (C6H6) at 80.00C to liquid benzene at 25.00C. Use Tables 8.1 and 8.2 for the specific heat, boiling point, and heat of vaporization of benzene.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER