
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
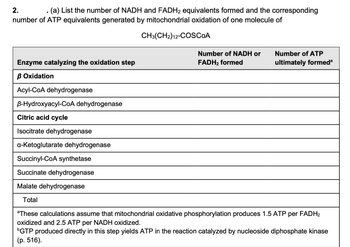
Transcribed Image Text:2.
(a) List the number of NADH and FADH2 equivalents formed and the corresponding
number of ATP equivalents generated by mitochondrial oxidation of one molecule of
CH3(CH2) 12-COSCOA
Number of NADH or
FADH₂ formed
Number of ATP
ultimately formedª
Enzyme catalyzing the oxidation step
B Oxidation
Acyl-CoA dehydrogenase
B-Hydroxyacyl-CoA dehydrogenase
Citric acid cycle
Isocitrate dehydrogenase
a-ketoglutarate dehydrogenase
Succinyl-CoA synthetase
Succinate dehydrogenase
Malate dehydrogenase
Total
aThese calculations assume that mitochondrial oxidative phosphorylation produces 1.5 ATP per FADH2
oxidized and 2.5 ATP per NADH oxidized.
GTP produced directly in this step yields ATP in the reaction catalyzed by nucleoside diphosphate kinase
(p. 516).
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- . FAD is a stronger oxidant than NAD*; FAD has a higher standard reduction potential than NAD*. Yet in the last reaction of the pyruvate dehydrogenase complex Explain this apparent paradox. FADH, bound to the Eg subunit is oxidized by NAD*.arrow_forwardWrite the equation for net citric acid cycle and calculate one cycle equivalent to how many ATP?arrow_forwardwhich statements are False?arrow_forward
- Which of the following is higher during high levels of glutathione reductase and NADP? SH A но (GSH) OH NH2 NH2 HO OH ;-CH-CH2-CH2-C-NH-CH–ċ–NH–CH2- CH2 В (GSSG) CH2 -CH-CH;-CH2=C-NH–C NH-CH–Ç-NH-CH2-C HO OH NH2arrow_forward9. The equation below depicts the first step of the citric acid cycle. H0 CoA-SH CH-C + 0=C-CO0 HO C-COO citrate s-COA ČH2-COO synthase ČH2 -CO0 Citrate Acetyl-CoA Oxaloacetate AG" = -32.2 kJ/mol a) Explain the chemical conversions that take place during this step. b) Why is this reaction energetically favorable? Explain.arrow_forwardAcetyi CoA Oxaloncetate CoA NADH NAD: Citrate Isccitrate Malate NAD co NADH Funaate »FADH; FAD a- Ketoglutarate Succinate NAD ATP Succinyt CuA NADH ADP - P, If you were told to add one of the eight citric acid cycle intermediates to the culture medium fo yeast growing in the laboratory, what do you think would happen to the rates of ATP and carbon dioxide production? (see the above figure) a. There would be no change in ATP production, but the rate of CO2 production would increase. b. The rates of ATP production and CO2 production would both increase, c. The rate of ATP production would increase, but the rate of CO2 production would decrease. d. The rates fo ATP and CO2 production would both decrease.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON