
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
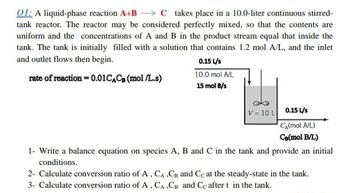
Transcribed Image Text:01: A liquid-phase reaction A+B C takes place in a 10.0-liter continuous stirred-
tank reactor. The reactor may be considered perfectly mixed, so that the contents are
uniform and the concentrations of A and B in the product stream equal that inside the
tank. The tank is initially filled with a solution that contains 1.2 mol A/L, and the inlet
and outlet flows then begin.
0.15 L/s
rate of reaction = 0.01 CACB (mol/L.s)
10.0 mol A/L
15 mol B/s
V = 10 L 0.15 L/s
CA(mol A/L)
CB(mol B/L)
1- Write a balance equation on species A, B and C in the tank and provide an initial
conditions.
2- Calculate conversion ratio of A, CA, CB and Cc at the steady-state in the tank.
3- Calculate conversion ratio of A, CA,CB and Cc after t in the tank.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps

Knowledge Booster
Similar questions
- The formation of NO from Na and O is to be carried out in a small batch reactor. As a first approximation, we shall consider that the reaction time is more rapid than the time of cylinder compression, consequently, the reaction takes place isothermally in car eylinder at 2700 K, in a constant volume reactor (cylinder) and under a pressure of 20 atm, the initial concentration of N2 is 0.0696 mol/liter. By a specifying constant volume, we are assuming that reaction take place rapidly with respect to the movement of the piston in the cylinder. Consider that the feed consists of 77% N2, 15% Oz and 8% other gases, which may be considered inert. At this temperature the equilibrium constant (Ke-0.01). The reaction is reversible: Na + 0z + 2NO With a rate equation: Cho -TN, = k ( CN, Co, Ke Calculate: a) The equilibrium conversion of N2. b) The time required to achieve 80% of the equilibrium conversion. The formation reaction rate- constant k at this temperature is 1.11 liter mol.h' Hint: dx…arrow_forwardA binary distillation is performed so that a distillate 94% and bottoms 8% in of the more volatile Component A results. The process shown below reflects one operating condition of a feed stream. Rectification, Stripping and the q-line are all provided on the x-y diagram below. Aling with expected stage for the distillation.. What is temperature of the liquid stream leaving the reboiler? What is the temperature of the vapor stream leaving the feed stage? What is the temperature of the liquid stream leaving stage 2? What is the temperature of the liquid reflux returned to the column? Note: A total condensor is installed.arrow_forwardThe following reaction takes place in a catalytic reactor: NO2+O2 → NO3 In the process 1000 mol/s of NO3 are produced. The reaction has 90% conversion and air with 20% excess is supplied. Calculate the flow rates of the system.arrow_forward
- -2 The hydrolysis of urea by urease occurs in a batch bioreactor. The reaction follows a Michaelis- Menten mechanism. The following kinetic data are available for urea: K'm=7.7×10 M, v =0.5 mole/L/min. The initial concentration of urea in the reactor is 50 mole/L. m E+S ES-²E + P Vm[S] Km + [S] Calculate the concentration of urea in the reactor 10 minutes after the reaction starts v= (Hint: it is a batch reactor, meaning that you need to integrate the MM, see module 1 for details of batch reactors).arrow_forwardIn a batch reactor, a substance A was processed, which generated different products (D and U), through competitive parallel reactions with the following reaction kinetics: After 20 minutes of reaction, it was determined that the composition of the reaction medium was CA = 1 mol/L, CD = 5 mol/L, CU = 2 mol/L. The option that indicates, respectively, the instantaneous and global selectivities at the end of the reaction are: A) 3 and 2. B) 2.5 and 2. C) 2 and 3. D) 2 and 2.5.arrow_forwardIn a continuous steady-state process the following two reactions take place: C6H12 + 6H2O à 6CO + 12H2 C6H12 + H2 à C6H14 In the process 150 moles of C6H12 and 400 moles of H2O are fed into reactor each hour. The yield of H2 is 50% and the selectivity of the first reaction compare to the second reaction is 22. Calculate the molar flow rates of the all five components in the output stream.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The