
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
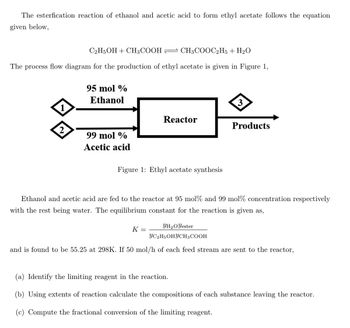
Transcribed Image Text:The esterfication reaction of ethanol and acetic acid to form ethyl acetate follows the equation
given below,
C2H5OH + CH3COOH CH3COOC2H5 + H₂O
The process flow diagram for the production of ethyl acetate is given in Figure 1,
1
2
95 mol %
Ethanol
99 mol %
Acetic acid
Reactor
Figure 1: Ethyl acetate synthesis
3
K =
Products
Ethanol and acetic acid are fed to the reactor at 95 mol% and 99 mol% concentration respectively
with the rest being water. The equilibrium constant for the reaction is given as,
YH₂O Yester
YC2H5OHYCH3 COOH
and is found to be 55.25 at 298K. If 50 mol/h of each feed stream are sent
the reactor,
(a) Identify the limiting reagent in the reaction.
(b) Using extents of reaction calculate the compositions of each substance leaving the reactor.
(c) Compute the fractional conversion of the limiting reagent.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 5 steps with 5 images

Knowledge Booster
Similar questions
- Normal butane is catalytically isomerized to isobutane. A fresh feed stream containing pure n-butane at temperature T, is mixed adiabatically with a recycle stream containing n-butane and isobutane, and the combined stream is fed to the reactor, where some but not all of the n-butane in the feed is converted. The reactor effluent is pumped to a distillation column. The overhead distillation product contains primarily isobutane and a small amount of n-butane. The bottoms product, which also contains both species, is the stream recycled to the reactor. The streams leaving the distillation column are at different temperatures. a) Determine the number of degrees of freedom associated with the feed mixer, the reactor, the distillation column, and the entire process. Include all unknown process stream temperatures and process unit heat duties in your analysis. b) Suppose a single-pass conversion of 35.0% is attained in the reactor, the overhead distillation column product contains 88.5 mole…arrow_forwardChemical Engineering Calculation All questions are interconnected, allowed by bartleby. PROBLEM #2: In a process for the manufacture of chlorine by direct oxidation of HCl with air over a catalyst to form Cl2 and H2O, the exit product is composed of 4.4% HCl, 19.8% Cl2, 19.8% H2O, 4% O2, and 52% N2. Detailed Solution in the Following: 0. Draw Process Diagram 1. What is the balanced chemical equation? 2. The limiting reactant is _________. 3. The percentage excess of the excess reactant is _______%. 4. What is the conversion of the limiting reactant? 5. What is the extent of reaction?arrow_forwardConsider the pair of reactions in which ethylene is oxidized either to ethylene oxide (desired) or to carbon dioxide (undesired) in the furnace: C₂H4+0₂-C₂H4O C₂H₂ +30₂-2 CO₂ + 2H₂O The feed mixture and air are fed at a temperature To. All gaseous effluents are at temperature T emerging from the non-isothermal reactor. a) Calculate the number of degrees of freedom of the process. How would the answer differ if the reactor were adiabatic? b) Outline a manual calculation procedure to determine the compositions of all streams.arrow_forward
- Pls do Asap...!arrow_forwardThe equilibrium constant with respect to temperature can be expressed for the reaction: Cyclohexane (g)! <--> methylcyclopentane (g) as: LnK = 4.814-2059/T. If placed to react 3 moles cyclohexane in a 5 liter container at a certain temperature and when equilibrium is reached, 8000 J of heat is released, Calculate the temperature of the container.arrow_forwardDirect dehydrogenation of ethylbenzene to styrene is carried out in the vapor phase with steam over a catalyst consisting primarily of iron oxide. The reaction is endothermic, and can be accomplished either adiabatically or isothermally. Both methods are used in practice. The major reaction is the reversible, endothermic conversion of ethylbenzene to styrene and hydrogen: C6H3CH₂CH CoHsCHCH₂ + H₂ AH= 124.9 kJ/mol Competing thermal reactions degrade ethylbenzene to benzene C6H3CH₂CH3C6H6+ C₂H4 AH 101.8 kJ/mol Styrene also reacts catalytically to toluene: CH3CH₂CH3 + H2 CH3CH3 + CH4 AH=64.5 kJ/mol The reactions take place at 620°C. The costs are as shown in Table 1. The production rate of styrene is 200 mol/h. Chemical name Formula Cost (S/kmol) Ethylbenzene C6H5CH₂CH3 57.1 Styrene C.HSCHCH₂ 75.9 Benzene C6H6 32.8 Toluene C6H5CH3 25.8 Hydrogen H₂ 1.2 (as fuel) Methane CH4 4.0 (as fuel) Ethylene C₂H4 6.7 (as fuel) Correlation for the product selectivity and distribution are given as…arrow_forward
- Alumina from bauxite separation stage calculations A crucial step in the production of aluminum from bauxite ore is the separation of alumina from the remaining mineral impurities in the ore. In the Bayer process this is accomplished by treating bauxite with aqueous NaOH to produce NaAIO2 (alumina). NaOH(aq) + Al(OH)3(s) → NaAIO2(aq) + 2H2O(1) Since NaAIO2 is water soluble while the residual mineral constituents of bauxite are not, a separation can be achieved by allowing the minerals to settle out and decanting the aqueous solution of NAAIO2 and unreacted NaOH. In order to further recover any NAAIO2 entrained in the settled mineral solids, this "mud" is repeatedly washed with water and allowed to settle, and the wash water is decanted. The figure below shows one stage of this washing-settling process. Wash water Slurry - Open to air Mixer/Wash tank Setling tank Decanted solution System boundary Washed mud In this stage, a feed slurry consisting of 10 wt% solids, 11 wt% NaOH, 16 wt%…arrow_forwardBasic Principles of Chemical Engineering Question Please solve step by steparrow_forwardOne of the ways that benzene is produced on a large scale is the hydrodealkylation of toluene. C,H,CH; + H, C,H, + CH4 A stream of toluene is mixed with a recycle stream and enters a reactor along with a stream of pure hydrogen. The reaction products at 550 °C enter a condenser, where they are cooled to 41.0 °C. A vapor stream containing Y5CH, = 0.600 mol CH,/mol leaves the process, and a liquid stream containing x66 = 0.810 mol benzene/mol and x6 = 0.190 mol toluene/mol enters a distillation column. The distillate of the column leaves the process at n7 = 668.0 mol/h and contains y7h = 0.9000 mol benzene/mol and y7 = 0.1000 mol toluene/mol. The bottoms of the column contains X8b = 0.250 mol benzene/mol and xgt = 0.750 mol toluene/mol and is recycled back to the fresh feed. Hydrogen is fed into the process at ni = 1183 mol H,/h. This process is carried out at 760 mmHg. A n, mol/h mol H/mol YSH2 YSCH, mol CH_/mol Ysh mol b/mol Ys mol t/mol n̟ mol/h n, mol/h Condenser Улн, тol H/mol Усн,…arrow_forward
- Nitromethane, CH3NO2 , can be used as a fuel. When the liquid is burned, the (unbalanced) reaction is mainlyCH3NO2(l) + O2(g) → CO2(g) + N2(g) + H2O(g)a. The standard enthalpy change of reaction (ΔH°van ) for the balanced reaction (with lowest whole-number coefficients) is −1288.5 kJ. Calculate ΔHf0 for nitromethane.b. A 15.0-L flask containing a sample of nitromethane is filled with O2 and the flask is heated to 100.°C. At this temperature, and after the reaction is complete, the total pressure of all the gases inside the flask is 950. torr. If the mole fraction of nitrogen (χnitrogen) is 0.134 after the reaction is complete, what mass of nitrogen was produced?arrow_forwardFor the electrolysis of CuCl2 (molten) to form Cu(s) and Cl2(g):E°Cu2+/Cu = 0.339 VE°Cl-/Cl2 = 1.360 V What minimum voltage must be used to carry out the reaction? If 1.5V is used, how much electrical energy (in kJ) will be used to produce 2g of Cl2(s)?arrow_forwardQuestion 13.6. The CO/H2 gas mixture is fed to a reactor at a ratio of ¹1/2 and heated under pressure to 650 K in the presence of a suitable catalyst. In order for this industrial reaction to be economically feasible, the equilibrium conversion value must be at least 30%. It can be assumed that the gas mixture exhibits ideal gas behavior in equilibrium. CO (g) + 2H₂(g) → CH3OH (g) In a reactor where the maximum working pressure value is 200 atm, the reaction in question whether it can be achieved in a way that meets the desired conditions. determine. If any problem arises in this regard, the equilibrium condition Possible improvements (if any) in order to meet the requirements put it forward. Use the average heat capacity ((CD)H) approach in your calculations.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The