
12.39 help
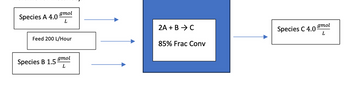
Problem 1) Sketch a process flow diagram for a continuous-stirred tank reactor where it is receiving a feed of 200 liters per hour and a reactant concentration of 4.0 gmoles/L for species A and 1.5 gmoles/L for species B. The reaction is 2A + B → C in the reactor is elementary and follows third-order kinetics for the overall reaction. Assume that the fractional conversion of the reactor is 85%. Label both the input streams and the output streams, listing all species that will be in each stream. Your quantities or variables in the diagram should be consistent in units of measurement (i.e. use only one unit of measurement to express volume for instance).
Problem 2) Write (but do not solve) the mole balance for the CSTR reactor in Problem 1 with respect to the limiting reactant in the system described above. Re-arrange this mole balance to show an expression for the CSTR’s volume. Assume that neither the reactor’s conversion nor the rate constant have been specified (leave them as variables).
please help w/ both if possible type answer i have included diagram that i created, not sure if its right
Step by stepSolved in 3 steps with 7 images

can you answer part 2 now
can you answer part 2 now
- Q2- The synthesis of ammonia proceeds according to the following reaction N₂ + 3 H₂ -----> 2 NH3 In a given plant, 4202 lb of nitrogen and 1046 lb of hydrogen are fed to the synthesis reactor per hour. Production of pure ammonia from this reactor is 3060 lb per hour. a. What is the limiting reactant. b. What is the percent excess reactant. c. What is the percent conversion obtained (based on the limiting reactant).arrow_forwardI have a reactor which is operating at steady state. The balanced reaction is shown below: 1A + 5 B => 2 M + 1 N The feed to the reactor contains only A and B, and B is fed in excess by 29 % of what is required to react all of the A. The reactor has a single outlet stream, and all unused reactants and all products leave the reactor in the same stream. The fractional conversion of the A in the reactor is 0.36. Calculate the mole fraction of M in the outlet from the reactor. Place your answer in the blank below, rounded to two decimal places.arrow_forward4-23. A mixture of ethanol (A) and water (B) is separated in a distillation column. The volumetric flow rate of the feed stream is 5 m'/hr. The concentration of ethanol in the feed is c,=2,800 mol/m'. The distillate leaves the column with a concentration of ethanol c, =13,000 mol/m. The volumetric flow rate of 3 distillate is one cubic meter per hour. How much ethanol is lost through the bottoms of the column, in kilograms of ethanol per hour?arrow_forward
- Consider the following potential energy diagram: * 1 p Reaction A Reaction B potential potential energy energy Reaction Pathway Reaction Pathway Reaction A is exothermic while Reaction B is endothermic Reaction A is endothermic while Reaction B is exothermic Reaction A will most likely be spontaneous while Reaction B is unlikely to be spontaneous O Reaction A releases heat while Reaction B absorbs heatarrow_forwardA) Construct a complete stoichiometric table for the molar flow rate and gas-phase concentrations using the correct limiting reactantarrow_forwardA binary distillation is performed so that a distillate 94% and bottoms 8% in of the more volatile Component A results. The process shown below reflects one operating condition of a feed stream. Rectification, Stripping and the q-line are all provided on the x-y diagram below. Aling with expected stage for the distillation.. What is temperature of the liquid stream leaving the reboiler? What is the temperature of the vapor stream leaving the feed stage? What is the temperature of the liquid stream leaving stage 2? What is the temperature of the liquid reflux returned to the column? Note: A total condensor is installed.arrow_forward
- The following reaction takes place in a catalytic reactor: NO2+O2 → NO3 In the process 1000 mol/s of NO3 are produced. The reaction has 90% conversion and air with 20% excess is supplied. Calculate the flow rates of the system.arrow_forwardIn a batch reactor, a substance A was processed, which generated different products (D and U), through competitive parallel reactions with the following reaction kinetics: After 20 minutes of reaction, it was determined that the composition of the reaction medium was CA = 1 mol/L, CD = 5 mol/L, CU = 2 mol/L. The option that indicates, respectively, the instantaneous and global selectivities at the end of the reaction are: A) 3 and 2. B) 2.5 and 2. C) 2 and 3. D) 2 and 2.5.arrow_forwardThe elementary gas-phase reaction A → 3B is carried out in a cylindrical batch reactor divided by a thin metallic membrane into two compartments of equal volume, as shown in the figure below. Pure A Pure I Initially one side is filled with pure 'A', and the other with an inert gas T. Both sides are at the same pressure (1 atm.), the same temperature, and contain the same number of moles. The reaction is carried out isothermally and the rate constant for the reaction is 0.1 min. a. After 10 minutes the membrane ruptures. What is the pressure differential rating of the membrane? What is the conversion at this time? b. If the mixture continues to react after the membrane is ruptured, how much longer does it take for the conversion to reach 90% (based on the initial moles of A)? c. Sketch the pressure versus time behavior for the entire duration. Mark all the important values on your diagram. HINT; Note that in each phase (before and after rupture) the reaction is occurring in a constant…arrow_forward
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





