
a)

Interpretation:
The product of the reaction shown if the reaction was conducted in DMSO with water is to be given. The complete mechanism of the reaction including appropriate regiochemistry and stereochemistry is also to be given.
Concept introduction:
The addition of halogens to
To give:
The product of the reaction shown if the reaction was conducted in DMSO with water and to give the complete mechanism of the reaction including appropriate regiochemistry and stereochemistry.
b)
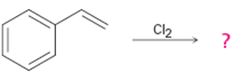
Interpretation:
The product of the reaction shown if the reaction was conducted in DMSO with water is to be given. The complete mechanism of the reaction including appropriate regiochemistry and stereochemistry is also to be given.
Concept introduction:
The addition of halogens to alkenes in the presence of aqueous DMSO results in the anti addition of the halohydrin, HOX, to the double bond. In the first step a cyclic halonium ion is formed by the attack of the double bond on the halogen. In the second step water attacks the halonium ion from the least shielded side to give an anti addition product. The addition obeys Markovnikov orientation. The negative part (OH) adds to the doubly bonded carbon atom which has more number of substituents.
To give:
The product of the reaction shown if the reaction was conducted in DMSO with water and to give the complete mechanism of the reaction including appropriate regiochemistry and stereochemistry.
c)
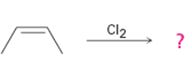
Interpretation:
The product of the reaction shown if the reaction was conducted in DMSO with water is to be given. The complete mechanism of the reaction including appropriate regiochemistry and stereochemistry is also to be given.
Concept introduction:
The addition of halogens to alkenes in the presence of aqueous DMSO results in the anti addition of the halohydrin, HOX, to the double bond. In the first step a cyclic halonium ion is formed by the attack of the double bond on halogen. In the second step water attacks the halonium ion from the least shielded side to give an anti addition product. The addition obeys Markovnokov orientation. The negative part (OH) adds to the doubly bonded carbon atom which has more number of substituents.
To give:
The product of the reaction shown if the reaction was conducted in DMSO with water and to give the complete mechanism of the reaction including appropriate regiochemistry and stereochemistry.
Trending nowThis is a popular solution!

Chapter 8 Solutions
Organic Chemistry
- Provide the mechanism and products for the acid-catalyzed epoxide opening reactions below, including appropriate stereochemistry.arrow_forwardUse your general knowledge of alkene chemistry to suggest a mechanism for the following reaction.arrow_forwardThe accepted mechanism for a rearrangement reaction is shown below. "Q Ph (1) (ii) Ph Ph HO™ fast Ph Ph HO O™ slow Ph aufae .Ar² LOH HO™ fast Draw a curly arrow mechanism for this transformation. Sketch an energy profile for this reaction, showing approximate relative energies for the reactants, intermediates, transition states, and products. HQ Ph (iii) When DO™ was used as the reagent, the rate of product formation was same as for HO™. Explain why this result shows that the third step is fast and cannot be rate-determining. Ph (iv) Write the predicted rate equation for this mechanism, and show how it was derived. (v) The starting diketone may have two different aryl groups, as shown below. Propose a ¹³℃ labelling experiment that would allow you to distinguish which of the two aryl groups had migrated in this situation. OH AR²0- Ar¹ C Carrow_forward
- biackboard.gwu.edu/webapPps/assessment/feview/review.jsprattempld Select the major product of the reaction below. 1) TIPSCI, Et3N 2) Na 3) 03, then CH3SCH3 4) CH3CH2LI, then H30* 5) Bu4N* F', H2O CI Selected Answer: Он HO Answers: он он он OH OH OH он Select the major product of the reaction below. The answer is there, but please explain step by step.arrow_forwardConsider the following reaction (not balanced): Benzaldehyde + HNO3/H2SO4 m-Nitrobenzaldehyde Write the step by step mechanism for the formation of m-Nitrobenzaldehyde. Show all appropriate arrows!!!arrow_forward5 a) For each of the reactions below, describe a suitable reaction mechanism, stating the reaction conditions and naming the major productsi) Nitrobenzene and Chloromethaneft) 2-chloro-2-methyl propane and aqueous potassium hydroxide.iii) Propanone and hydrogen cyanide.iv) iodoethane and sodium hydroxide in ethanol.b) i) Discuss the bonding and extra.stability of benzene,ii) Using suitable examples, compare the reactivity of benzene and ethane,arrow_forward
- 13.a. Give the mechanism(s) and product(s) for the reaction below. Show stereochemistry. CH3 H3C CH3 ||||C KOtBu tBuOHarrow_forwarducf.edu/courses/1379813/quizzes). Question 12 Identify the major product from the following series of reactions. 1. NaOEt 1. NaOEt 2. CH3CH2CI OEt 2. H30*, Quendch 3. H30*, Heat но он HO H.arrow_forward9. Write structural formulas for the major organic product(s) for the following reactions. Be sure to indicate stereochemistry when relevant. (CH;CH,CH,),CuLi (a) Cl Br PAL2 (b) HO (c) CH;CH,CH,CH,OH C,H5MgBr 10. Propose a reasonable mechanism for the following reaction Be sure to include all intermediates. (transition states are not necessary) Br Br hv CH3-CH=C(CH3)2 + Br, ČH,-CH=C(CH3)2 CH2=CH–Ċ(CH3)2 11. Indicate how each of the following compounds could be prepared using the given starting material CH;CH,CH,CH3 CH;CH,CH,CH,Br CH;CH,CH,CH3 H,C=CH-CH=CH2 Br CH;CHCH,CH3 CH;CH=CCH,CH3 CH3arrow_forward
- Please fill in the blanks!! Which set of reagents (given below) would produce the products given in the following reaction? OH OH ? A E В C D Br2, H20 Br2, DCM HBr, DCM HBr, H20 HBr, H2O2 G H I J F NBS H2, Pd 03, DMS KMNO4 1. Li, 2. Cul K L M heat Grubb's CH212, Znarrow_forwardPredict the major organic product for the following reaction sequence. Be sure your answer accounts for stereochemistry and regiochemistry, whe appropriate. If multiple stereoisomers are formed, be sure to draw all products using appropriate wedges and dashes. H₂C CH₂ 1. BH3, THF 2. H2O2, NaOH, H₂O 3. PB13 4. (CH3CH2CH2)2CuLi ?arrow_forward10. Predict the products of the following reaction and write a reaction mechanism. I ㅋ Br2 ? Cl₂ ?arrow_forward
