
a)
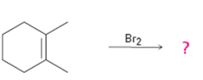
Interpretation:
The product formed with its stereochemistry, when 1,2-dimethylcyclohexene reacts with Br2 is to be given. The mechanism of its formation also is to be given.
Concept introduction:
The reaction of halogens to
To give:
The product expected, along with its stereochemistry, when Br2 is added to 1,2-dimethylcyclohexene.
b)
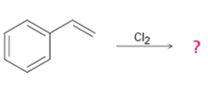
Interpretation:
The product obtained with its stereochemistry, in the reaction shown, is to be given. The mechanism of its formation also is to be given.
Concept introduction:
The reaction of halogens to alkenes occurs with anti stereochemistry, that is, the two halogen atoms come from opposite faces of the double bond, one from top face and other from bottom face. In the first step, the addition of halogen to the double bond in the alkenes results in the formation of a cyclic halonium ion with the simultaneous elimination of a halide ion. The large halonium ion shields one side of the molecule. Hence the attack of the halide ion occurs from the opposite, unshielded side to yield a trans product.
To give:
The product expected, along with its stereochemistry, when Cl2 is added to styrene (vinyl benzene).
c)
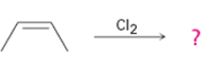
Interpretation:
The product obtained with its stereochemistry in the reaction shown is to be given. The mechanism of its formation also is to be given.
Concept introduction:
The reaction of halogens to alkenes occurs with anti stereochemistry, that is, the two halogen atoms come from opposite faces of the double bond- one from top face and other from bottom face. In the first step, the addition of halogen to the double bond in the alkenes results in the formation of a cyclic halonium ion with the simultaneous elimination of a halide ion. The large halonium ion shields one side of the molecule. Hence the attack of the halide ion occurs from the opposite, unshielded side to yield a trans-product.
To give:
The product expected, along with its stereochemistry, when Cl2 is added to 2-butene.
Trending nowThis is a popular solution!

Chapter 8 Solutions
Organic Chemistry
- Aldehydes and ketones react with thiols to yield thioacetals just as they react with alcohols to yield acetals. Predict the product of the following reaction, and propose a mechanism:arrow_forwardIdentify the following pericyclic reaction; explain the course and stereochemistry of the reaction.arrow_forwardPredict the products and show the mechanisms for the following reactions. Please indicate the correct stereochemistry where necessary.arrow_forward
- Predict the products of the following reaction, including stereochemistry. 1) OsO4 2) NaHSO, Write the mechanism and predict the products of the following reaction, including stereochemistry. Br2, H,Oarrow_forwardProvide the structure of the product(s) with stereochemisty if appropriate. If multiple products indicate major product or if products are formed in equal amounts. If there is no reaction expected explain why. (a) OH HIO4 (the protecting group is remained) CHO H HOH2C OH (b) CHO -ОН NaCN # HO -H H -OH CH₂OH (c) CHO HO- -H HO -H NH₂OH 張一 H -OH H -OH CH₂OH i) Ba(OH)2 ii) MeOH, H₂SO4 NaOAc Ac₂0 NaOMe MeOHarrow_forwardPredict the product(s) of the following reactions, including stereochemistry when necessary and identify the mechanism of each substitution reaction (SN1 vs SN2). Draw the reaction mechanism (reaction arrows) for any one of the reactions to show how the product is formed.arrow_forward
- Predict the organic products, in any order, when the following alkene is subjected to ozonolysis with reductive work-up Products:arrow_forwardPredict the products of the following reactions.Indicate the stereochemistry in the products when relevant.arrow_forwardPredict the products of each reaction below. Indicate stereochemistry when relevant.arrow_forward
- Predict the products and indicate the relative stereochemistry for the following transformations. When necessary indicate the major productarrow_forwardPredict the main organic product including any possible stereochemistry for the following reactions. LIAIH 4 COOCH CH,arrow_forwardDraw all of the substitution and elimination products formed from thegiven alkyl halide with each reagent: (a) CH3OH; (b) KOH. Indicate thestereochemistry around the stereogenic centers present in the products,as well as the mechanism by which each product is formed.arrow_forward
