
Concept explainers
Interpretation:Whether in the model of 1,2-dimethylcyclopentane, the molecule in the left box is same as the molecule in the right box or not needs to be determined.
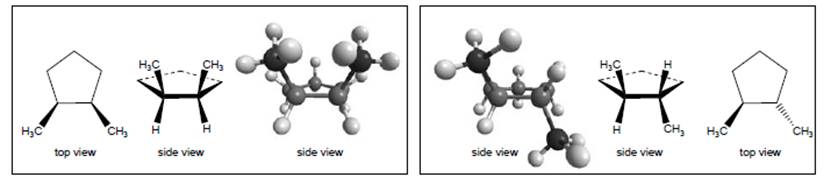
Concept Introduction:
The cis and trans isomerism concept will be applied here. Any molecule will be called cis if it follows the conditions for itthat is the same groups attached either above the plane or below the plane.For trans isomer,same groups are attached where one is above the plane and other group is below the plane or vice versa.
Answer to Problem 1CTQ
The given both structures of isomers of 1,2 dimethylcyclopentane are similar to each other. This is because when the bonds of the groups interchanges with each other, the bond’s spatial character changes. As a result of which these structures can be converted to each other without breaking of bonds.
Explanation of Solution
It is given that the left box and right box contain two molecules of 1,2-dimethylcyclopentane.Thus, it is very easy to conclude that the 2 different molecules are isomers of 1,2-dimethylcyclopentane. This is because both the molecules have same number of atoms in it.
Both the molecules are similar toeach other. Thiscan be explained as, if the interchanging of bonds takes place, similar structure can be obtained. In the interchanging of structure in left box and right box, there is no breaking of bonds takes place. For example, if the methyl group attached to the bond below the planeis interchanged with other group attached to the same atom, then the position of methyl group will be converted to above the plane from below the plane. In this process, no breaking of bond takes place then similar structure will be obtained.
Thus, the structure on the left box is similar to that on the right box.
Want to see more full solutions like this?
Chapter 7 Solutions
Organic Chemistry: A Guided Inquiry
- Image below, Why is this not a constructual isomer?arrow_forwardUsing your model, construct an energy diagram to show the variation in the free energy of the molecule as the FRONT ATOM is rotated CLOCKWISE from 0º to 360º in 60º increments. In your energy diagram, you should clearly show the relative energies of each conformer.arrow_forwardCould we cut just one bond in the "starting" molecule shown in the drawing area below to create this "target" molecule? The target molecule. If so, highlight the bond to be cut. If not, check the box under the drawing area that says Not possible. Note: it's OK if cutting the bond creates more than one molecule, as long as one of them is the target molecule. Not possible. X H H-C-H H-O-H H 3 Note for advanced students: what we mean by "cutting" the bond here is breaking the bond and attaching H atoms to each dangling end, like this: H H-C-0-H Harrow_forward
- a) Draw one isomer of C6H14. b) Draw one isomer of C6H12- c) Draw one isomer of C6H140 that exhibits hydrogen bonding. d) Draw one isomer of C6H140 that is not capable of hydrogen bonding. BONUS: Show all locations of possible hydrogen bonding for the C6H140 isomer that you drew above in part c.arrow_forwardCould we cut just one bond in the "starting" molecule shown in the drawing area below to create this "target" molecule? The target molecule. If so, highlight the bond to be cut. If not, check the box under the drawing area that says Not possible. Note: it's OK if cutting the bond creates more than one molecule, as long as one of them is the target molecule. Xarrow_forwardCheck the box under each structure in the table that is an enantiomer of the molecule shown below. If none of them are, check the none of the above box under the table. OH & .... Molecule 1 HO Molecule 4 OH -- none of the above Molecule 2 HO In.. Molecule 5 HO X Molecule 3 sil OH Molecule 6 ..... OH X Ś 000 Ar 18arrow_forward
- Could we cut just one bond in the "starting" molecule shown in the drawing area below to create this "target" molecule? The target molecule. If so, highlight the bond to be cut. If not, check the box under the drawing area that says Not possible. Note: it's OK if cutting the bond creates more than one molecule, as long as one of them is the target molecule. 5arrow_forwardWithout counting hydrogens, determine which one of the following CANNOT be the unknownmolecule with molecular formula C7H8NOBr , and explain your reasoning.arrow_forwarda model of each molecule shown above: Is the molecule in the left box the same moleculeas the molecule in the right box? Use your models to answer the question, and recall that...arrow_forward
- I don't understand why the first one and second one are cis and trans respectively. Wouldn't the first one be trans-1,2-dimethylcyclobutane because the torsional strain wouldn't allow the carbons to be in the same conformation. Meaning one of the carbons would be up and then the next would be down and so on. Since both methyl groups are equatorial and the first and second carbons are arranged up and down, wouldn't it be trans. Same logic for the second molecule. Carbon 1, which is attached to the methyl is down, carbon 3 which is attached to the methyl should be down also because of torsional strain, and since both methyl are in axial (or equatorial?), it would be cis. Or is it based off of the way the carbons are positioned in the picture?arrow_forward3. Draw the dash-wedge structure for the following molecules. 1 C₂H₂ C₂H₂ S H CH₂ 1 H Br H Cl * H H 4. Arrange the structures in the increasing order of stability CH₂ C₂H₂ C₂M₂ C₂H₂ H C₂H₂ *馬來西 H C₂H₂ H C₂H₂ CHy CH₂ IV || |||arrow_forwardChoose the correct answer. Choose.. Choose.. A saturated hydrocarbon with molecular formula C6H14 An alkyne with molecular formula C5H10 A ketone with molecular formula C4H80 An alkene with molecular formula C5H10 An aldehyde with molecular formula CH4O An alkene with molecular formula C5H8 A ketone with molecular formula C3H8O An aldehyde with molecular formula C2H4O Choose..arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
