
Chemistry: An Atoms First Approach
2nd Edition
ISBN: 9781305079243
Author: Steven S. Zumdahl, Susan A. Zumdahl
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 21, Problem 141CWP
a. Name each of the following alcohols.
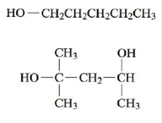
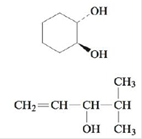
b. Name each of the following alcohols, including the stereochemistry if cis-trans isomers are possible.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
1.
a. Draw the structures of the two isomers of dibromoethane. Name them by the IUPAC system.
b. Draw out the structures of the two isomers of iodopropane. Name them by the IUPAC system.
c. Draw the structures of the three isomers of dichloroethene. Name each one by the IUPAC system, indicating stereochemistry where relevant.
C. Nomenclature of Cylic Alkanes:
If a ring is present in a molecule, the ring is almost always considered the parent chain.
Use the word cyclo as a prefix before the alkane name to indicate it is a cyclic parent chain
Number the carbons that bear substituents starting with one, and number around the ring towards the closest carbon
that bears another substituent group to give all substituents lowest possible numbering.
If there are two or more substituents, use alphabetical order to determine where to start numbering.
Critical Thinking Question (CTQ):
11. Give the correct IUPAC name for the remaining three compounds below.
H,C
CH-CH,
l-isopropyl-3-methylcycloherane
12. Draw the structures that correspond to the following names:
a. 3-ethylheptane
b. 1,1-diethylcyclopropane
c. 4-sec-butyl-1-isobutyl-2-methylcylcohexane
13. Mono-substituted cycloalkanes do not include the "1" locant. Thus, 1-methylcyclohexane is inaccurate.
Draw methylcyclohexane; explain why adding a locant does not add…
Which of the following is true of conformational isomers?
O A. They arise from rotation about single bonds.
B. They cannot be isolated under ordinary conditions of temperature and pressure.
O C. They have the same structural formulas
D. all of these
E. none of these
Chapter 21 Solutions
Chemistry: An Atoms First Approach
Ch. 21 - What is a hydrocarbon? What is the difference...Ch. 21 - Prob. 2RQCh. 21 - Prob. 3RQCh. 21 - Summarize the nomenclature rules for alkanes,...Ch. 21 - What functional group distinguishes each of the...Ch. 21 - Distinguish between isomerism and resonance....Ch. 21 - Prob. 7RQCh. 21 - Prob. 8RQCh. 21 - Prob. 9RQCh. 21 - Prob. 10RQ
Ch. 21 - Prob. 11RQCh. 21 - Prob. 12RQCh. 21 - Prob. 1QCh. 21 - Prob. 2QCh. 21 - What is wrong with the following names? Give the...Ch. 21 - Prob. 4QCh. 21 - Prob. 5QCh. 21 - Prob. 6QCh. 21 - Prob. 7QCh. 21 - Prob. 8QCh. 21 - Prob. 9QCh. 21 - Prob. 10QCh. 21 - Prob. 11QCh. 21 - Prob. 12QCh. 21 - Prob. 13ECh. 21 - Prob. 14ECh. 21 - Draw all the structural isomers for C8H18 that...Ch. 21 - Draw all the structural isomers for C8H18 that...Ch. 21 - Prob. 17ECh. 21 - Prob. 18ECh. 21 - Draw the structural formula for each of the...Ch. 21 - Prob. 20ECh. 21 - Prob. 21ECh. 21 - Prob. 22ECh. 21 - Prob. 23ECh. 21 - Prob. 24ECh. 21 - Name each of the following alkenes. a. CH2 = CH ...Ch. 21 - Name each of the following alkenes or alkynes. a....Ch. 21 - Prob. 27ECh. 21 - Prob. 28ECh. 21 - Prob. 29ECh. 21 - Prob. 30ECh. 21 - Name each of the following. a. b. CH3CH2CH2CCl3 c....Ch. 21 - Prob. 32ECh. 21 - There is only one compound that is named...Ch. 21 - Prob. 34ECh. 21 - Prob. 35ECh. 21 - Prob. 36ECh. 21 - Prob. 37ECh. 21 - Prob. 38ECh. 21 - Prob. 39ECh. 21 - Prob. 40ECh. 21 - Draw all structural and geometrical (cistrans)...Ch. 21 - Prob. 42ECh. 21 - Prob. 43ECh. 21 - Prob. 44ECh. 21 - If one hydrogen in a hydrocarbon is replaced by a...Ch. 21 - There are three isomers of dichlorobenzene, one of...Ch. 21 - Prob. 47ECh. 21 - Prob. 48ECh. 21 - Prob. 49ECh. 21 - Minoxidil (C9H15N5O) is a compound produced by...Ch. 21 - Prob. 51ECh. 21 - Prob. 52ECh. 21 - Name all the alcohols that have the formula...Ch. 21 - Prob. 54ECh. 21 - Prob. 55ECh. 21 - Prob. 56ECh. 21 - Prob. 57ECh. 21 - Prob. 58ECh. 21 - Prob. 59ECh. 21 - Prob. 60ECh. 21 - Prob. 61ECh. 21 - Prob. 62ECh. 21 - Prob. 63ECh. 21 - Prob. 64ECh. 21 - Prob. 65ECh. 21 - Prob. 66ECh. 21 - Prob. 67ECh. 21 - Prob. 68ECh. 21 - Prob. 69ECh. 21 - Complete the following reactions. a. CH3CO2H +...Ch. 21 - Prob. 71ECh. 21 - Prob. 72ECh. 21 - Prob. 73ECh. 21 - Prob. 74ECh. 21 - Prob. 75ECh. 21 - The polyester formed from lactic acid, is used for...Ch. 21 - Prob. 77ECh. 21 - Prob. 78ECh. 21 - Prob. 79ECh. 21 - Prob. 80ECh. 21 - Prob. 81ECh. 21 - Prob. 82ECh. 21 - Prob. 83ECh. 21 - Prob. 84ECh. 21 - Prob. 85ECh. 21 - Prob. 86ECh. 21 - Prob. 87ECh. 21 - Prob. 88ECh. 21 - Prob. 89ECh. 21 - Prob. 90ECh. 21 - Prob. 91ECh. 21 - Prob. 92ECh. 21 - Prob. 93ECh. 21 - Prob. 94ECh. 21 - Prob. 95ECh. 21 - Prob. 96ECh. 21 - Prob. 97ECh. 21 - Prob. 98ECh. 21 - Prob. 99ECh. 21 - Prob. 100ECh. 21 - Prob. 101ECh. 21 - Prob. 102ECh. 21 - Prob. 103ECh. 21 - Prob. 104ECh. 21 - Prob. 105ECh. 21 - Prob. 106ECh. 21 - Which base will hydrogen-bond with uracil within...Ch. 21 - Prob. 108ECh. 21 - The base sequences in mRNA that code for certain...Ch. 21 - Prob. 110ECh. 21 - Prob. 111AECh. 21 - Prob. 112AECh. 21 - Prob. 113AECh. 21 - Prob. 114AECh. 21 - Prob. 115AECh. 21 - Prob. 116AECh. 21 - Prob. 117AECh. 21 - Prob. 118AECh. 21 - Prob. 119AECh. 21 - Prob. 120AECh. 21 - Prob. 121AECh. 21 - Prob. 122AECh. 21 - Prob. 123AECh. 21 - Prob. 124AECh. 21 - Prob. 125AECh. 21 - Prob. 126AECh. 21 - Prob. 127AECh. 21 - Prob. 128AECh. 21 - Prob. 129AECh. 21 - Prob. 130AECh. 21 - Prob. 131AECh. 21 - Prob. 132AECh. 21 - Prob. 133AECh. 21 - Prob. 134AECh. 21 - When heat is added to proteins, the hydrogen...Ch. 21 - Prob. 136AECh. 21 - Prob. 137CWPCh. 21 - Prob. 138CWPCh. 21 - Prob. 139CWPCh. 21 - Name each of the following alkenes and alkynes. a....Ch. 21 - a. Name each of the following alcohols. b. Name...Ch. 21 - Prob. 142CWPCh. 21 - Prob. 143CWPCh. 21 - Prob. 144CWPCh. 21 - Prob. 145CPCh. 21 - Prob. 146CPCh. 21 - Prob. 147CPCh. 21 - Prob. 148CPCh. 21 - Prob. 149CPCh. 21 - Prob. 150CPCh. 21 - Prob. 151CPCh. 21 - Prob. 152CPCh. 21 - Prob. 153CPCh. 21 - Prob. 154CPCh. 21 - Stretch a rubber band while holding it gently to...Ch. 21 - Alcohols are very useful starting materials for...Ch. 21 - Prob. 157CPCh. 21 - Prob. 158CPCh. 21 - Prob. 159IPCh. 21 - Prob. 160IPCh. 21 - Prob. 161MPCh. 21 - Prob. 162MP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 1. In dash-wedge-line structure, the dashes represent the: * A. bonds in the plane of the page. B. bonds away from observer. C. bonds towards observer. D. bonds in the plane of the paper. 2. Which of the following is an ACYCLIC SATURATED hydrocarbon? * A. 1-Ethyl-3-methylcyclopentane B. 3-Ethyl-3-methylpentane C. 1,3,5,7-Octatetraene D. 5-Methyl-1,3-cyclopentadiene 3. Which of the following is a CYCLIC SATURATED hydrocarbon? * A. 3-Ethyl-4-methylcyclohexene B. 3,5-Diethyloctane C. 2,2-Dimethyl-3-heptyne D. 3-Butyl-1,1-dimethyl-5-propylcyclohexanearrow_forward13. The alkyl radical formed when a hydrogen atom is being removed from the second carbon of a normal four-carbon alkane is always a) a normal alkyl. 14. Disubtituted benzene compound with two attached groups that are opposite to each other is a) a meta isomer. 15. Two alkyl groups that are attached to the same C atom which has a double-bonded O atom is a) an aldehyde. b) an isoalkyl. c) a secondary alkyl. d) a tertiary alkyl. b) a para isomer. c) an ortho isomer. d) a beta isomer. b) an alcohol. c) a carboxylic acid. d) a ketone. 16. Which of the following organic compounds are not capable of hydrogen bonding by themselves? a) ketones. 17. The hydrocarbon which can undergo substitution bromination reaction. a) alkane 18. The minor product between the unsymmetrical butene and HBr is a) 1,2- dibromobutane. 19. Which of the following amines does not belong to the same group? a) aniline c) dimethylamine b) carboxylic acids c) amines d) alcohols b) alkene c) alkyne d) cycloalkene b)…arrow_forwardHexane and cyclohexane are examples of two molecules that are. A . Constitutional Isomers B. cis - trans isomers C. identical D. enanitiomers E . Unrelated with different formulasarrow_forward
- Identify the organic functional groups and reaction type for the following reaction if only the major product is shown.The reactant is a(n)a. alkyneb. etherc. alkened. alcohole. aromaticThe product is a(n)a. tertiary alcoholb. primary alcoholc. aldehyded. ethere. secondary alcoholThe reaction type is :a. hydrolysisb. hydrationc. reduction (hydrogenation)d. dehydratione. oxidationarrow_forwardHow many stereoisomers are possible for the molecule formed by the reaction described in the preceding question? Group of answer choices a. 8 b. 16 c. 32 d. 64 (preceding question is image #2)arrow_forward3. Draw all the isomers (including structural and geometric isomers) of CH;Br; which contain a carbon-carbon double bond. 4. This question is about the effect of geometric isomerism on the melting and boiling points of geometric isomers. If you haven't done this bit yet, ignore the question. a) Explain why the boiling point of cis-1,2-dichloroethene is higher than that of the trans isomer. b) Explain why the melting point of cis-1,2-dichloroethene is lower than that of the trans isomer.arrow_forward
- Identify the organic functional groups and reaction type for the following reaction if only the major product is shown.The reactant is a(n)a. alkyneb. etherc. alkened. alcohole. aromaticThe product is a(n)a. tertiary alcoholb. primary alcoholc. aldehyde d. ethere. secondary alcoholThe reaction type is :a. hydrolysisb. hydrationc. reduction (hydrogenation)d. dehydratione. oxidationI got alkene, tertiary alcohol, and hydrolysis, but I missed one of them. What are the correct answers?arrow_forwardOrganic Chemistry 15. ADLC Assignment Booklet A3 Decide whether each statement is true (T) or false (F). Place your answer in the blank space given. a. A hydrocarbon molecule with a triple bond is more stable than a hydrocarbon molecule with all single bonds. b. The difference between butter and corn oil is that the hydrocarbon molecule in butter contains a double carbon- carbon bond. c. A bent shape is the property that accounts for many of the healthy properties of oleic acid present in corn oil. d. A diet with no fat is the healthiest diet. e. In an industrially produced trans fat, only the relative position of the hydrogen atoms on either side of the double bond is changed. f. Trans fatty acids have a greater likelihood of clogging arteries because they tend to be solids at room temperature.arrow_forwardSome alkenes have cis-trans isomers because a. the carbon atoms in the double bond are free to rotate. b. one of the carbon atoms in the double bond has two identical groups attached to it. c. all of the carbon atoms in the compound are rigid and cannot rotate. d. each of the carbon atoms in the double bond has four different groups attached to it. e. the carbon atoms in the double bond cannot rotate.arrow_forward
- 1. Identify the SYSTEMATIC name of the aliphatic hydrocarbon a. 1-pentenyicyclopentane b. 1-cyclopentylpent-2-yne c. 1-pentenecyclopent-2-yne d. 1-pentenyicyclopentane 2. Identify the SYSTEMATIC name of the aliphatic hydrocarbon a. Trans-1,2-propylcyclopropane b. Trans-1,2-diisopropylcyclopropane c. Cis-1,2-propylcyclopropane d. Cis-1,2-diisopropylcyclopropane 3. Identify the SYSTEMATIC name of the aliphatic hydrocarbon 3,10-dimethyl-2-decacen-6-yne 3,10-dimethyl-2-dodocen-6-yne 3,10-dimethyl-10-decacen-6-yne d. 3,10-dimethyl-10-dodocen-6-yne a. b. C. 4. Identify which type of isomer the following structures represent HO a. Skeletal Isomer b. E/Z isomer c. Cis/Trans isomer OH OH d. Positional isomer 5. Identify which type of isomer the following structures represent a. Skeletal Isomer Br b. E/Z isomer c Cis/Trans isomer Br d. Positional isomerarrow_forward6. Any organic compound that contains a benzene ring or similar feature. O a. addition reaction Ob. aliphatic compound Oc. alkene d. alkyne Oe. aromatic hydrocarbon Of. hydration g. hydrogenation h. monomer Oi. phenyl group j. polycyclic aromatic hydrocarbon k. polymer I. unsaturated hydrocarbonarrow_forward13. Ethylethanoate and butanoic acid can be classified as A. positional isomers B. chain isomers C. functional isomers D. stereoisomers 14. Which of the following pairs are positional isomers A. trans-1,4-dichlorocyclohexane, cis-1,3-dichlorocyclopentane B. trans 1,4-dichlorocyclohexane, cis-1,4-dichlorocyclohexane C. 2-pentanol, Cyclopentanol D. 1,2-cycohexanediol, 1,3-cycohexanediol 15. Which of the following compounds will have zero dipole moment? A. cis-1,2-dibromoethylene B. 1,1-dibromoethylene C. trans-1,2-dibromoethylene D. all of these 16. Which of the following is not aromatic: A. cyclopentadienyl cation B. cyclopentadienyl anion C. Cyclopropenyl cation D. Cycloheptatrienyl cation 17. Which of the following compounds containing lone pair has the least tendency to donate its electrons? A. the lone pair in pyridine B. the lone pair in furan C. the lone pair in pyrole D. the lone pair in thiophenearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License