
(a)
Interpretation:
For the given molecule, the IUPAC is to be written.
Concept introduction:
An ester consists of a
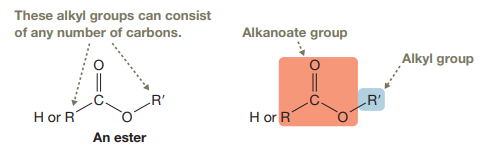
(b)
Interpretation:
For the given molecule, the IUPAC is to be written.
Concept introduction:
An ester consists of an

(c)
Interpretation:
For the given molecule, the IUPAC is to be written.
Concept introduction:
An ester consists of an

Trending nowThis is a popular solution!

Chapter F Solutions
Organic Chemistry: Principles and Mechanisms (Second Edition)
- Each of the following names is wrong. Give the structure and correct name of each compound. a 3-ethyl-2-methylbutanal b 2-ethyl-4-butanone c.4, 5-dibromocyclopentanonearrow_forwardWhat are the IUPAC Name and Molecular formula of the structure?arrow_forwardProvide the correct IUPAC name for each compound below. b.arrow_forward

 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning


