
Organic Chemistry Plus Masteringchemistry With Pearson Etext, Global Edition
9th Edition
ISBN: 9781292151229
Author: Wade, LeRoy G.
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 9, Problem 9.41SP
The following functional-group interchange is a useful synthesis of

- a. What reagents were used in this chapter for this transformation? Give an example to illustrate this method.
- b. This functional-group interchange can also be accomplished using the following sequence.
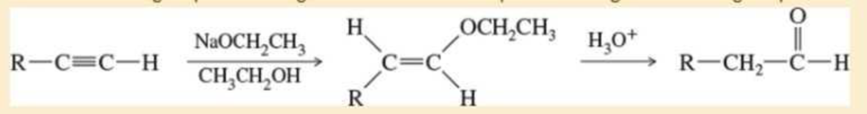
Propose mechanisms for these steps.
- c. Explain why a nucleophilic reagent such as ethoxide adds to an
alkyne more easily than it adds to analkene .
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
By means of series of equations, show how each of the following synthesis can be carried out. You may
use any other reagents and more than one step will be necessary.
a. cis-1,2-cyclopentanediol from cyclopentanol
b.
0 from cyclohexyl bromide
c. cvclopentvl methanol from cyclopentanol
e. CH;CH,CH from n-propyl bromide
OH
4. Provide a synthetic route to the following molecule using benzene and cyclohexane.
Reagents cannot contain more than one carbon. Provide a mechanism for the last step
of your synthetic route.
A reaction flask contains 2-bromopentane in an ethanolic solution of sodium ethoxide at room temperature and result in the formation of two olefnic products. What is responsible for the formation of major and minor products. A. Different activated complex involved in the mechanism. B. Bimolecular nucleophilic substitution reaction. C. Bimolecular elimination reaction D. The presence of sodium ethoxide. E. The hybridization nature of secondary carbocation
Chapter 9 Solutions
Organic Chemistry Plus Masteringchemistry With Pearson Etext, Global Edition
Ch. 9.1 - a. Count the elements of unsaturation in...Ch. 9.2 - Prob. 9.2PCh. 9.4B - What reaction would acetylene likely undergo if it...Ch. 9.6 - Prob. 9.4PCh. 9.6 - Predict the products of the following acid-base...Ch. 9.7A - Solved Problem9-1 showed the synthesis of...Ch. 9.7A - Show how you might synthesize the following...Ch. 9.7B - Prob. 9.8PCh. 9.7B - Show how you would synthesize...Ch. 9.8 - When 2,2-dibromo-1-phenylpropane is heated...
Ch. 9.8 - When 2,2-dibromo-1-phenylpropane is heated...Ch. 9.9C - Show how you would convert a. oct-3-yne to...Ch. 9.9C - The fragrance of (Z)-1-phenylhex-2-en-1-ol...Ch. 9.9D - In the addition of just 1 mole of bromine to 1...Ch. 9.9E - Propose a mechanism for the entire reaction of...Ch. 9.9E - Predict the major product(s) of the following...Ch. 9.9E - Propose a mechanism for the reaction of pent-1-yne...Ch. 9.9E - Show how hex-1-yne might be converted to a....Ch. 9.9F - When pent-2-yne reacts with mercuric sulfate in...Ch. 9.9F - Prob. 9.20PCh. 9.9F - Prob. 9.21PCh. 9.9F - Prob. 9.22PCh. 9.10A - Predict the product(s) you would expect from...Ch. 9.10B - Prob. 9.24PCh. 9.10B - Prob. 9.25PCh. 9 - Prob. 9.26SPCh. 9 - Give common names for the following compounds. a....Ch. 9 - Prob. 9.28SPCh. 9 - Prob. 9.29SPCh. 9 - Using cyclooctyne as your starting material, show...Ch. 9 - Prob. 9.31SPCh. 9 - Prob. 9.32SPCh. 9 - Predict the products of reaction of pent-1-yne...Ch. 9 - Show how you would accomplish the following...Ch. 9 - Show how you would synthesize the following...Ch. 9 - Predict the products formed when CH3CH2C C : Na+...Ch. 9 - Prob. 9.37SPCh. 9 - Prob. 9.38SPCh. 9 - When compound Z is treated with ozone, followed by...Ch. 9 - Show how you would convert the following starting...Ch. 9 - The following functional-group interchange is a...Ch. 9 - Using any necessary inorganic reagents, show how...Ch. 9 - Prob. 9.43SP
Additional Science Textbook Solutions
Find more solutions based on key concepts
Give the IUPAC name for each compound.
Organic Chemistry
The active ingredient in Tylenol and a host of other over-the-counter pain relievers is acetaminophen (C8H9NO2)...
Chemistry: Atoms First
141. Design a device that uses as electrochemical cell to determine amount of
in a sample water Describe, in...
Chemistry: Structure and Properties (2nd Edition)
Q1. What is the empirical formula of a compound with the molecular formula
Chemistry: A Molecular Approach
What is the pH range for acidic solutions? For basic solutions?
EBK INTRODUCTION TO CHEMISTRY
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Propose a mechanism for the following reaction. a. Explain why two products are formed. b. Explain why methanol substitutes for only one of the brominesarrow_forwardPropose reagents and conditions for the following multistep syntheses. You may use any reagents necessary. Be sure to draw all isolable intermediates. Br. H.arrow_forward1. Show all steps in the synthesis of 4-methylaniline from toluene. Clearly show all reagents, reactants and products for each step. Toluene -------> --------> 4-methylanilinearrow_forward
- Give the products of the following substitution reactions. For every reaction, show electron pairs on both nucleophile and leaving group.arrow_forwardShow what reagents you would use to synthesize this ether by each of the following methods, show mechanism for method A and C. A. Acid-catalyzed ether formation from alcohols B. Alkoxymercuration-demercuration (do not show mechanism) C. Williamson ether synthesisarrow_forward6. Multi Step Synthesis. Propose a synthetic route from the reactant to the product using any reagents you need. Nalt LINH4arrow_forward
- Choose all correct statements. 1. Gilman reagents react with alkyl halides, including vinyl and aryl halides. 2. Grignard reagents react with alkyl halides, including vinyl and aryl halides. 3. Deprotonation of an alcohol group forms an oxonium intermediate. 4. Whenever a neutral nucleophile attacks, a proton transfer step follows. 5. An alcohol that is chiral at the a-carbon will undergo inversion of absolute configuration when it is converted into an alkyl tosylate. C 1 2 3 4arrow_forwardShow how you would synthesize each compound from benzene, toluene, or phenol using the following reactions: Reactions 1. Halogenation 2. Nitration 3. Sulfonation 4. Friedel-Crafts acylation 5. Friedel-Crafts alkylation 6. Oxidation of methyl group 7. Reduction of nitro group • Choose the starting material from the drop-down list • Enter the number(s) of the desired reactions in the order that you wish to use them without commas between • If a reaction is used more than once, enter its number each time you wish to use it Compound 1: 5-chloro-2-methylbenzenesulfonic acid Starting material: toluene Reaction(s): 31 Compound 2: m-bromobenzoic acid Starting material: [benzene Reaction(s): Submit Answer Try Another Version 1 item attempt remainingarrow_forwardAssume that attached starting materials can be converted to an epoxide by this reaction. Draw the product formed (including stereochemistry) from each starting material. Why might some of these reactions be more difficult than others in yielding nucleophilic substitution products?arrow_forward
- What reagents would you require for this reaction?arrow_forwardFrom benzene and compounds of 4 carbons or less and any reagents you want; synthesize the following compound.arrow_forward4. Propose an efficient synthetic route (along with intermediates) for the following transformation. Note: Use cyclohexane as the starting point for both cyclohexyl rings in the product.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Coenzymes and cofactors; Author: CH15 SWAYAM Prabha IIT Madras;https://www.youtube.com/watch?v=bubY2Nm7hVM;License: Standard YouTube License, CC-BY
Aromaticity and Huckel's Rule; Author: Professor Dave Explains;https://www.youtube.com/watch?v=7-BguH4_WBQ;License: Standard Youtube License