
Organic Chemistry Plus Masteringchemistry With Pearson Etext, Global Edition
9th Edition
ISBN: 9781292151229
Author: Wade, LeRoy G.
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 9, Problem 9.30SP
Using cyclooctyne as your starting material, show how you would synthesize the following compounds. (Once you have shown how to synthesize a compound, you may use it as the starting material in any later parts of this problem.)
- a. cis-cyclooctene
- b. cyclooctane
- c. trans-1,2-dibromocyclooctane
- d. cyclooctanone
- e. 1,1-dibromocyclooctane
- f. 3-bromocyclooctene
- g. cyclooctane- 1,2-dione
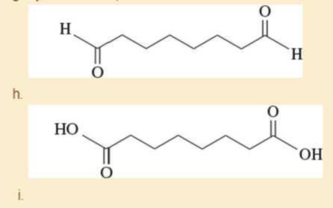
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Show how to convert propene to each of these compounds, using any inorganic reagents as necessary.
a. Propane
b.1,2-Propanediol
c. 1-Propanol
d. 2-Propanol
e. Propanal
f. Propanone
g. Propanoic acid
h. l-Bromo-2-propanol
i. 3-Chloropropene
j. 1,2,3-Trichloropropane
k. 1-Chloropropane
l. 2-Chloropropane
m. 2-Propen-1-ol
n. Propenal
1. 2-bromobutane + cyclohexanol + NaH à (major product)
c. 2-butoxycyclohexane
a.. 2-butene
reaction
b. 1-butene
d. no
e. something else!
2. t-butylbromide + sodium ethoxide in ethanol à (major product)
a. 2-methylpropene
reaction
b. t-butyl ethyl ether
c. 1-methylbutene
d. no
e. something else!
3. potassium t-butoxide + 1-bromobutane in t-butyl alcohol room
temperature à (major product)
a. 1-butene
reaction
c. 2-methylpropene d. no
b. butyl t-butyl ether
e. something else!
4. t-butyl bromide + boiling hot water à (major product)
a. 2-methylpropene
reaction
b. t-butyl ethyl ether
c. t-butanol
d. no
e. something else!
5. 2-chloropropane + acetic acid (2 eq) / KOH (1 eq) / DMF à(major
product)
c.
b.
d. no reaction
e. something
а.
HO.
else!
Br
KOH / DMSO
6.
b. 2-methyl-1-propanol
d. 1,2-
a. 2-methylpropene
propanediene
c. no reaction
e. something else!
7. cyclopentanol+ NaH + DMSO + bromopropane à(major product)
a. cyclopentene
reaction
b. propene
e. something else!!
c. propyl cyclopentyl…
Draw the structure for each compound.
a. (R)-3-methylhexane
b. (4R,5S)-4,5-diethyloctane
c. (3R,5S6R)-5-ethyl-3,6-dimethylnonane
d. (3S,6S)-6-isopropyl-3-methyldecane
Chapter 9 Solutions
Organic Chemistry Plus Masteringchemistry With Pearson Etext, Global Edition
Ch. 9.1 - a. Count the elements of unsaturation in...Ch. 9.2 - Prob. 9.2PCh. 9.4B - What reaction would acetylene likely undergo if it...Ch. 9.6 - Prob. 9.4PCh. 9.6 - Predict the products of the following acid-base...Ch. 9.7A - Solved Problem9-1 showed the synthesis of...Ch. 9.7A - Show how you might synthesize the following...Ch. 9.7B - Prob. 9.8PCh. 9.7B - Show how you would synthesize...Ch. 9.8 - When 2,2-dibromo-1-phenylpropane is heated...
Ch. 9.8 - When 2,2-dibromo-1-phenylpropane is heated...Ch. 9.9C - Show how you would convert a. oct-3-yne to...Ch. 9.9C - The fragrance of (Z)-1-phenylhex-2-en-1-ol...Ch. 9.9D - In the addition of just 1 mole of bromine to 1...Ch. 9.9E - Propose a mechanism for the entire reaction of...Ch. 9.9E - Predict the major product(s) of the following...Ch. 9.9E - Propose a mechanism for the reaction of pent-1-yne...Ch. 9.9E - Show how hex-1-yne might be converted to a....Ch. 9.9F - When pent-2-yne reacts with mercuric sulfate in...Ch. 9.9F - Prob. 9.20PCh. 9.9F - Prob. 9.21PCh. 9.9F - Prob. 9.22PCh. 9.10A - Predict the product(s) you would expect from...Ch. 9.10B - Prob. 9.24PCh. 9.10B - Prob. 9.25PCh. 9 - Prob. 9.26SPCh. 9 - Give common names for the following compounds. a....Ch. 9 - Prob. 9.28SPCh. 9 - Prob. 9.29SPCh. 9 - Using cyclooctyne as your starting material, show...Ch. 9 - Prob. 9.31SPCh. 9 - Prob. 9.32SPCh. 9 - Predict the products of reaction of pent-1-yne...Ch. 9 - Show how you would accomplish the following...Ch. 9 - Show how you would synthesize the following...Ch. 9 - Predict the products formed when CH3CH2C C : Na+...Ch. 9 - Prob. 9.37SPCh. 9 - Prob. 9.38SPCh. 9 - When compound Z is treated with ozone, followed by...Ch. 9 - Show how you would convert the following starting...Ch. 9 - The following functional-group interchange is a...Ch. 9 - Using any necessary inorganic reagents, show how...Ch. 9 - Prob. 9.43SP
Additional Science Textbook Solutions
Find more solutions based on key concepts
How could you separate a mixture of the following compounds? The reagents available to you are water, either, 1...
Organic Chemistry
Draw a Lewis structure for each of the following species: a. H2CO3 b. CO32 c. CH2O d. CO2
Essential Organic Chemistry (3rd Edition)
141. Design a device that uses as electrochemical cell to determine amount of
in a sample water Describe, in...
Chemistry: Structure and Properties (2nd Edition)
Problem 11.1 Neopheliosyne B is a novel acetylenic fatty acid isolated from a New Caledonian marine sponge. (a)...
Organic Chemistry
1. What did each of the following scientists contribute to our knowledge of the atom?
a. William Crookes
b. E...
Chemistry For Changing Times (14th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the structures for the following molecules. Write your answers on clean scratch papers and upload it after you've taken the exam. A. (R)-pentane-1,2,4-triol B. Cyclopentyloxycycloheptane C. (1R,2R)-Cyclohexane-1,2-diol D. Isobutyl phenyl ether (hint: phenyl is a benzene ring acting as a substituent) E. 2-methyl-3-propylpentane-1,5-diol F. 2-ethyl-3-isopropylbutane-1,4-diolarrow_forward1. Fill in the boxes with the missing reagents or major organic products for the alkene reactions. a. HCl b. H2O, H2SO4 с. d. 1. ВН, 2. H2O2, NaOH е. f. CI .CI g. Cl2, H2O h. 1. Н-О, Hg(OАс)2 2. NaBH4, NaOH i. 1. O3 2. Н:0, H-02 j. OH HO.arrow_forwardGive the structure corresponding to each name. a.3-chloro-2-methylhexane b.4-ethyl-5-iodo-2,2-dimethyloctane c.cis-1,3-dichlorocyclopentane d.1,1,3-tribromocyclohexane e. 6-ethyl-3-iodo-3,5-dimethylnonane f.(R)-1-fluoro-2,6,6-trimethylnonanearrow_forward
- Give the structure corresponding to each name. a. 3-chloro-2-methylhexane b. 4-ethyl-5-iodo-2,2-dimethyloctane c. cis-1,3-dichlorocyclopentane d. 1,1,3-tribromocyclohexane e. propyl chloride f. sec-butyl bromidearrow_forwardReagents a. C6H5CHO b. NaOH, ethanol h. BrCH2CH=CH2 i. Na* OEt, ethanol j. Br2, H* k. K* t-BuO c. Pyrrolidine, cat. H* d. H2C=CHCN e. H3O* f. I. CH2(CO2ET)2 -CH2CH2CN LDA m. heat g. ELOC(=0)CO2ET Select reagents from the table to synthesize this compound from cyclopentanone. Enter the letters of the chosen reagents, in the order that you wish to use them, without spaces or punctuation (i.e. geda).arrow_forwardDescribe (meaning what you would do and observe) simple (not including melting points, boiling points, cleaving reactions, or instrumental analyses) chemical tests(if any) that would distinguish between the compounds named below. [hint doing a table may be helpful] A. 1,3-pentadiene and 1-pentyne B. Ally bromide and 2,3- dimethyl-1,3-butadienearrow_forward
- Draw the structures of the molecules with the following names. a. 4,4-Dichloro-trans-2-octene b. (Z)-4-bromo-2-iodo-2-pentene c. 5-Methyl-cis-3-hexen-1-ol d. (R)-1,3-Dichlorocycloheptene e. (E)-3-Methoxy-2-methyl-2-buten-1-olarrow_forwardThe dehydrochlorination of 3-chloro-2-methylpentane in the presence of (CH3)3N, Ag2O and heat produces A. 2-methypent-2-ene B. 2-methypentane C. 2-methypent-3-ene D. 4-methypent-2-ene Which of the following species is electron repelling? A. –N2 B. –S- C. –Br D. –OHarrow_forwardDescribe (meaning what you would do and observe) simple (not including melting points, boiling points, cleaving reactions, or instrumental analyses) chemical tests (if any) that would distinguish between the compounds named below. [ Hint: using a table may be helpful ] A. 1,3-pentadiene & 1-pentyne B. allyl bromide & 2,3-dimethyl-1,3-butadienearrow_forward
- Functional groups such as alkynes react the same in complex molecules as they do in simpler structures. The following example of alkyne reaction were taken from syntheses carried out in the research group of E. J. Corey at Harvard University. You can assume that the reactions listed involve only the alkyne, not any of the functional groups present in the molecules. Draw the expected products for the following reaction .arrow_forwardTreatment of 1,2-dimethylcyclopentene with Pt and H2 gas will produce a.cis-1,2-dimethylcyclopentane. b.1,1-dimethylcyclopentane. c.trans-1,2-dimethylcyclopentane. d.mixture of trans and cis-1,2-dimethylcyclopentane.arrow_forwardWhat is the major product when 2-iodopentane is used in dehydrohalogenation reaction. A. 2-iodo-1-pentene B. 2-pentene C. (E)-2-pentene D. (Z)-2-pentene E. (Cis)-2-pentenearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
How to Design a Total Synthesis; Author: Chemistry Unleashed;https://www.youtube.com/watch?v=9jRfAJJO7mM;License: Standard YouTube License, CC-BY