
Thermodynamics: An Engineering Approach
9th Edition
ISBN: 9781259822674
Author: Yunus A. Cengel Dr., Michael A. Boles
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 8.8, Problem 77P
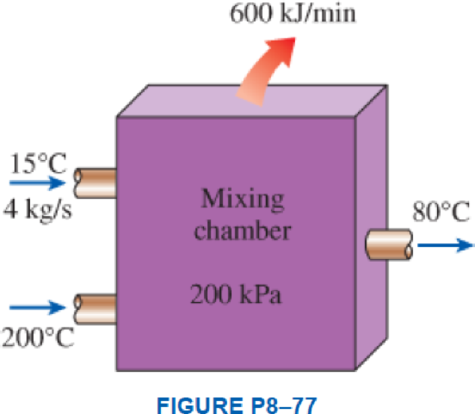
Liquid water at 200 kPa and 15°C is heated in a chamber by mixing it with superheated steam at 200 kPa and 200°C. Liquid water enters the mixing chamber at a rate of 4 kg/s, and the chamber is estimated to lose heat to the surrounding air at 25°C at a rate of 600 kJ/min. If the mixture leaves the mixing chamber at 200 kPa and 80°C, determine (a) the mass flow rate of the superheated steam and (b) the wasted work potential during this mixing process.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Student Name:
Student Id:
College of Applied Engineering
Al-Muzahmiyah Branch
Statics (AGE 1330) Section-1483
Quiz-2
Time: 20 minutes
Date: 16/02/2025
Q.1. A swinging door that weighs w=400.0N is supported by
hinges A and B so that the door can swing about a vertical'
axis passing through the hinges (as shown in below figure).
The door has a width of b=1.00m and the door slab has a
uniform mass density. The hinges are placed symmetrically
at the door's edge in such a way that the door's weight is
evenly distributed between them. The hinges are separated
by distance a=2.00m. Find the forces on the hinges when
the door rests half-open. Draw Free body diagram also.
[5 marks]
[CLO 1.2]
Mool
b
ర
a
2.0 m
B
1.0 m
For the walking-beam mechanism shown in Figure 3, find and plot the x and y coordinates of the
position of the coupler point P for one complete revolution of the crank O2A. Use the coordinate
system shown in Figure 3. Hint: Calculate them first with respect to the ground link 0204 and
then transform them into the global XY coordinate system.
y
-1.75
Ꮎ
Ꮎ
4
= 2.33
0242.22
L4
x
AP = 3.06
L2 = 1.0
W2
31°
B
03 L3 = 2.06
P
1
8
5
.06
6
7
P'
The link lengths, gear ratio (2), phase angle (Ø), and the value of 02 for some geared five bar
linkages are defined in Table 2. The linkage configuration and terminology are shown in Figure
2. For the rows assigned, find all possible solutions for angles 03 and 04 by the vector loop
method. Show your work in details: vector loop, vector equations, solution procedure.
Table 2
Row
Link 1 Link 2
Link 3
Link 4
Link 5
λ
Φ
Ө
a
6
1
7
9
4
2
30°
60°
P
y 4
YA
B
b
R4
R3
YA
A
Gear ratio:
a
02
d
05
r5
R5
R2
Phase angle: = 0₂-202
R1
05
02
r2
Figure 2.
04
X
Chapter 8 Solutions
Thermodynamics: An Engineering Approach
Ch. 8.8 - What final state will maximize the work output of...Ch. 8.8 - Is the exergy of a system different in different...Ch. 8.8 - Under what conditions does the reversible work...Ch. 8.8 - How does useful work differ from actual work? For...Ch. 8.8 - How does reversible work differ from useful work?Ch. 8.8 - Is a process during which no entropy is generated...Ch. 8.8 - Consider an environment of zero absolute pressure...Ch. 8.8 - It is well known that the actual work between the...Ch. 8.8 - Consider two geothermal wells whose energy...Ch. 8.8 - Consider two systems that are at the same pressure...
Ch. 8.8 - Prob. 11PCh. 8.8 - Does a power plant that has a higher thermal...Ch. 8.8 - Prob. 13PCh. 8.8 - Saturated steam is generated in a boiler by...Ch. 8.8 - One method of meeting the extra electric power...Ch. 8.8 - A heat engine that receives heat from a furnace at...Ch. 8.8 - Consider a thermal energy reservoir at 1500 K that...Ch. 8.8 - A heat engine receives heat from a source at 1100...Ch. 8.8 - A heat engine that rejects waste heat to a sink at...Ch. 8.8 - A geothermal power plant uses geothermal liquid...Ch. 8.8 - A house that is losing heat at a rate of 35,000...Ch. 8.8 - A freezer is maintained at 20F by removing heat...Ch. 8.8 - Prob. 24PCh. 8.8 - Prob. 25PCh. 8.8 - Prob. 26PCh. 8.8 - Can a system have a higher second-law efficiency...Ch. 8.8 - A mass of 8 kg of helium undergoes a process from...Ch. 8.8 - Which is a more valuable resource for work...Ch. 8.8 - Which has the capability to produce the most work...Ch. 8.8 - The radiator of a steam heating system has a...Ch. 8.8 - A well-insulated rigid tank contains 6 lbm of a...Ch. 8.8 - A pistoncylinder device contains 8 kg of...Ch. 8.8 - Prob. 35PCh. 8.8 - Prob. 36PCh. 8.8 - Prob. 37PCh. 8.8 - A pistoncylinder device initially contains 2 L of...Ch. 8.8 - A 0.8-m3 insulated rigid tank contains 1.54 kg of...Ch. 8.8 - An insulated pistoncylinder device initially...Ch. 8.8 - Prob. 41PCh. 8.8 - An insulated rigid tank is divided into two equal...Ch. 8.8 - A 50-kg iron block and a 20-kg copper block, both...Ch. 8.8 - Prob. 45PCh. 8.8 - Prob. 46PCh. 8.8 - Prob. 47PCh. 8.8 - A pistoncylinder device initially contains 1.4 kg...Ch. 8.8 - Prob. 49PCh. 8.8 - Prob. 50PCh. 8.8 - Prob. 51PCh. 8.8 - Air enters a nozzle steadily at 200 kPa and 65C...Ch. 8.8 - Prob. 54PCh. 8.8 - Prob. 55PCh. 8.8 - Argon gas enters an adiabatic compressor at 120...Ch. 8.8 - Prob. 57PCh. 8.8 - Prob. 58PCh. 8.8 - The adiabatic compressor of a refrigeration system...Ch. 8.8 - Refrigerant-134a at 140 kPa and 10C is compressed...Ch. 8.8 - Air enters a compressor at ambient conditions of...Ch. 8.8 - Combustion gases enter a gas turbine at 900C, 800...Ch. 8.8 - Steam enters a turbine at 9 MPa, 600C, and 60 m/s...Ch. 8.8 - Refrigerant-134a is condensed in a refrigeration...Ch. 8.8 - Prob. 66PCh. 8.8 - Refrigerant-22 absorbs heat from a cooled space at...Ch. 8.8 - Prob. 68PCh. 8.8 - Prob. 69PCh. 8.8 - Air enters a compressor at ambient conditions of...Ch. 8.8 - Hot combustion gases enter the nozzle of a...Ch. 8.8 - Prob. 72PCh. 8.8 - A 0.6-m3 rigid tank is filled with saturated...Ch. 8.8 - Prob. 74PCh. 8.8 - Prob. 75PCh. 8.8 - An insulated vertical pistoncylinder device...Ch. 8.8 - Liquid water at 200 kPa and 15C is heated in a...Ch. 8.8 - Prob. 78PCh. 8.8 - Prob. 79PCh. 8.8 - A well-insulated shell-and-tube heat exchanger is...Ch. 8.8 - Steam is to be condensed on the shell side of a...Ch. 8.8 - Prob. 82PCh. 8.8 - Prob. 83PCh. 8.8 - Prob. 84PCh. 8.8 - Prob. 85RPCh. 8.8 - Prob. 86RPCh. 8.8 - An aluminum pan has a flat bottom whose diameter...Ch. 8.8 - Prob. 88RPCh. 8.8 - Prob. 89RPCh. 8.8 - A well-insulated, thin-walled, counterflow heat...Ch. 8.8 - Prob. 92RPCh. 8.8 - Prob. 93RPCh. 8.8 - Prob. 94RPCh. 8.8 - Prob. 95RPCh. 8.8 - Nitrogen gas enters a diffuser at 100 kPa and 110C...Ch. 8.8 - Prob. 97RPCh. 8.8 - Steam enters an adiabatic nozzle at 3.5 MPa and...Ch. 8.8 - Prob. 99RPCh. 8.8 - A pistoncylinder device initially contains 8 ft3...Ch. 8.8 - An adiabatic turbine operates with air entering at...Ch. 8.8 - Steam at 7 MPa and 400C enters a two-stage...Ch. 8.8 - Prob. 103RPCh. 8.8 - Steam enters a two-stage adiabatic turbine at 8...Ch. 8.8 - Prob. 105RPCh. 8.8 - Prob. 106RPCh. 8.8 - Prob. 107RPCh. 8.8 - Prob. 108RPCh. 8.8 - Prob. 109RPCh. 8.8 - Prob. 111RPCh. 8.8 - A passive solar house that was losing heat to the...Ch. 8.8 - Prob. 113RPCh. 8.8 - A 4-L pressure cooker has an operating pressure of...Ch. 8.8 - Repeat Prob. 8114 if heat were supplied to the...Ch. 8.8 - Prob. 116RPCh. 8.8 - A rigid 50-L nitrogen cylinder is equipped with a...Ch. 8.8 - Prob. 118RPCh. 8.8 - Prob. 119RPCh. 8.8 - Prob. 120RPCh. 8.8 - Reconsider Prob. 8-120. The air stored in the tank...Ch. 8.8 - Prob. 122RPCh. 8.8 - Prob. 123RPCh. 8.8 - Prob. 124RPCh. 8.8 - Prob. 125RPCh. 8.8 - Prob. 126RPCh. 8.8 - Prob. 127RPCh. 8.8 - Water enters a pump at 100 kPa and 30C at a rate...Ch. 8.8 - Prob. 129RPCh. 8.8 - Prob. 130RPCh. 8.8 - Obtain a relation for the second-law efficiency of...Ch. 8.8 - Writing the first- and second-law relations and...Ch. 8.8 - Prob. 133RPCh. 8.8 - Keeping the limitations imposed by the second law...Ch. 8.8 - Prob. 135FEPCh. 8.8 - Prob. 136FEPCh. 8.8 - Prob. 137FEPCh. 8.8 - Prob. 138FEPCh. 8.8 - A furnace can supply heat steadily at 1300 K at a...Ch. 8.8 - A heat engine receives heat from a source at 1500...Ch. 8.8 - Air is throttled from 50C and 800 kPa to a...Ch. 8.8 - Prob. 142FEPCh. 8.8 - A 12-kg solid whose specific heat is 2.8 kJ/kgC is...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- Problem 4 A .025 lb bullet C is fired at end B of the 15-lb slender bar AB. The bar is initially at rest, and the initial velocity of the bullet is 1500 ft/s as shown. Assuming that the bullet becomes embedded in the bar, find (a) the angular velocity @2 of the bar immediately after impact, and (b) the percentage loss of kinetic energy as a result of the impact. (c) After the impact, does the bar swing up 90° and reach the horizontal? If it does, what is its angular velocity at this point? Answers: (a). @2=1.6 rad/s; (b). 99.6% loss = (c). Ah2 0.212 ft. The bar does not reach horizontal. y X 4 ft 15 lb V₁ 1500 ft/s 0.025 lb C 30°7 B Aarrow_forwardsubject: combustion please include complete solution, no rounding off, with diagram/explanation etc. In a joule cycle, intake of the compressor is 40,000 cfm at 0.3 psig and 90 deg F. The compression ratio is 6.0 and the inlet temperature at the turbine portion is 1900R while at the exit, it is 15 psi. Calculate for the back work ratio in percent.arrow_forwardsubject: combustion please include complete solution, no rounding off, with diagram/explanation etc. A gasoline engine, utilizing cold air, recorded a work of 431 BTU/lb at a maximum temperature of 3,273 K and 1112 deg F temperature at the beginning of constant volume heat addition. What is the compression ratio?arrow_forward
- subject: combustion please do step by step solution and no rounding off, complete solution with diagram/explanation if needed etc. thank you! Air enters the compressor at 101,320 Pascals, 305.15K, and leaves at a pressure of 0.808MPa. The air is heated to 990.15K in the combustion chamber. For a net output of 2,125,000 Watts, find the rate of flow of air per second.arrow_forwardThe link lengths and the value of 2 and offset for some fourbar crank-slide linkages are defined in Table 1. The linkage configuration and terminology are shown in Figure 1. For the rows assigned, find (a) all possible solutions for angle & and slider position d by vector loop method. (b) the transmission angle corresponding to angle 03. (Hint: Treat the vector R4 as virtual rocker) Show your work in details: vector loop, vector equations, solution procedure. Table 1 Row Link 2 Link 3 Offset Ө a 1.4 4 1 45° b 3 8 2 -30° C 5 20 -5 225° 03 slider axis B X offset Link 2 A R3 Link 3 R4 04 R2 02 R1 d Figure 1. Xarrow_forward4. Two links made of heat treated 6061 aluminum (Sy = 276 MPa, Sys = 160 MPa) are pinned together using a steel dowel pin (Sy = 1398 MPa, Sys = 806 MPa) as shown below. The links are to support a load P with a factor of safety of at least 2.0. Determine if the link will fail first by tearout, direct shear of the pin, bearing stress on the link, or tensile stress at section AA. (Hint: find the load P for each case and choose the case that gives the smallest load.) P 8 mm P 8 mm ¡+A 3 mm →A 10 mm Parrow_forward
- 1. For a feature other than a sphere, circularity is where: A. The axis is a straight line B. The modifier is specified with a size dimension C. All points of the surface intersected by any plane perpendicular to an axis or spine (curved line) are equidistant from that axis or spine D. All points of the surface intersected by any plane passing through a common center are equidistant from that center 2. What type of variation is limited by a circularity toler- ance zone? A. Ovality B. Tapering C. Bending D. Warping 3. How does the Rule #1 boundary affect the application of a circularity tolerance? A. The modifier must be used. B. The feature control frame must be placed next to the size dimension. C. The circularity tolerance value must be less than the limits of size tolerance. D. Circularity cannot be applied where a Rule #1 boundary exists. 4. A circularity tolerance may use a modifier. A. Ø B. F C. M D. ℗ 5. A real-world application for a circularity tolerance is: A. Assembly (i.e.,…arrow_forward3. A steel bar is pinned to a vertical support column by a 10 mm diameter hardened dowel pin, Figure 1. For P = 7500 N, find: a. the shear stress in the pin, b. the direct bearing stress on the hole in the bar, c. the minimum value of d to prevent tearout failure if the steel bar has a shear strength of 175 MPa. support column pin bar thickness of bar = 8 mm h d 150 mmarrow_forwardA press that delivers 115 strokes per minute, each stroke providing a force of 7826 N throughout a distance of 18 mm. The press efficiency is 90% and is driven by a 1749-rpm motor. Determine average torque that must be provided by the motor in the units of N-m.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY
What is entropy? - Jeff Phillips; Author: TED-Ed;https://www.youtube.com/watch?v=YM-uykVfq_E;License: Standard youtube license