
Concept explainers
Interpretation: The preferred resonance form among the given ions is to be stated on the basis of formal charges.
Concept introduction: Formal charges play an important role in choosing between the possible molecular structures. The preferred structure is the one in which formal charges are zero.
To determine: The preferred resonance form among the given ions on the basis of formal charges.
Answer to Problem 8.92QP
Solution
The preferred resonance form in the
The preferred resonance form in the
The preferred resonance form in the
Explanation of Solution
Explanation
The given ion is
Therefore the total valence electrons are
The lone pair of electrons on oxygen and bonding electrons is delocalized which results in the formation of lewis structures. The lewis structures for

Figure 1
The formal charge on each atom of resonating structure (a) of
Formal charge is calculated as,
Number of valence electrons in nitrogen is
Number of lone pair electrons in nitrogen is
Number of bond pair electrons in nitrogen is
To calculate the formal charge on nitrogen, substitute the value of valence electrons, lone pair electrons and bond pair electrons in the equation (1).
Number of valence electrons in oxygen is
Number of lone pair electrons in oxygen is
Number of bond pair electrons in oxygen is
To calculate the formal charge on oxygen, substitute the value of valence electrons, lone pair electrons and bond pair electrons in the equation (1).
Number of valence electrons in carbon is
Number of lone pair electrons in carbon is
Number of bond pair electrons in carbon is
To calculate the formal charge on carbon, substitute the value of valence electrons, lone pair electrons and bond pair electrons in the equation (1).
The formal charge on each atom of resonating structure (b) of
Number of valence electrons in nitrogen is
Number of lone pair electrons in nitrogen is
Number of bond pair electrons in nitrogen is
To calculate the formal charge on nitrogen, substitute the value of valence electrons, lone pair electrons and bond pair electrons in the equation (1).
Number of valence electrons in oxygen is
Number of lone pair electrons in oxygen is
Number of bond pair electrons in oxygen is
To calculate the formal charge on oxygen, substitute the value of valence electrons, lone pair electrons and bond pair electrons in the equation (1).
Number of valence electrons in carbon is
Number of lone pair electrons in carbon is
Number of bond pair electrons in carbon is
To calculate the formal charge on carbon, substitute the value of valence electrons, lone pair electrons and bond pair electrons in the equation (1).
According to formal charge calculations, there is a formal charge of
The given ion is
Therefore the total valence electrons are
Since carbon is least electronegative, it will act as central atom. The lone pair of electrons on oxygen and bonding electrons is delocalized which results in the formation of lewis structures. The lewis structures for
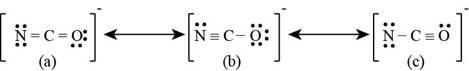
Figure 2
The formal charge on each atom of resonating structure (a) of
Number of valence electrons in nitrogen is
Number of lone pair electrons in nitrogen is
Number of bond pair electrons in nitrogen is
To calculate the formal charge on nitrogen, substitute the value of valence electrons, lone pair electrons and bond pair electrons in the equation (1).
Number of valence electrons in oxygen is
Number of lone pair electrons in oxygen is
Number of bond pair electrons in oxygen is
To calculate the formal charge on oxygen, substitute the value of valence electrons, lone pair electrons and bond pair electrons in the equation (1).
Number of valence electrons in carbon is
Number of lone pair electrons in carbon is
Number of bond pair electrons in carbon is
To calculate the formal charge on carbon, substitute the value of valence electrons, lone pair electrons and bond pair electrons in the equation (1).
The formal charge on each atom of resonating structure (b) of
Number of valence electrons in nitrogen is
Number of lone pair electrons in nitrogen is
Number of bond pair electrons in nitrogen is
To calculate the formal charge on nitrogen, substitute the value of valence electrons, lone pair electrons and bond pair electrons in the equation (1).
Number of valence electrons in oxygen is
Number of lone pair electrons in oxygen is
Number of bond pair electrons in oxygen is
To calculate the formal charge on oxygen, substitute the value of valence electrons, lone pair electrons and bond pair electrons in the equation (1).
Number of valence electrons in carbon is
Number of lone pair electrons in carbon is
Number of bond pair electrons in carbon is
To calculate the formal charge on carbon, substitute the value of valence electrons, lone pair electrons and bond pair electrons in the equation (1).
The formal charge on each atom of resonating structure (c) of
Number of valence electrons in nitrogen is
Number of lone pair electrons in nitrogen is
Number of bond pair electrons in nitrogen is
To calculate the formal charge on nitrogen, substitute the value of valence electrons, lone pair electrons and bond pair electrons in the equation (1).
Number of valence electrons in oxygen is
Number of lone pair electrons in oxygen is
Number of bond pair electrons in oxygen is
To calculate the formal charge on oxygen, substitute the value of valence electrons, lone pair electrons and bond pair electrons in the equation (1).
Number of valence electrons in carbon is
Number of lone pair electrons in carbon is
Number of bond pair electrons in carbon is
To calculate the formal charge on carbon, substitute the value of valence electrons, lone pair electrons and bond pair electrons in the equation (1).
According to formal charge calculations, there is a formal charge of
The given ion is
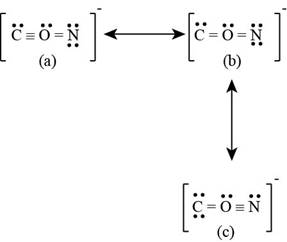
Figure 3
The formal charge on each atom of resonating structure (a) of
Number of valence electrons in nitrogen is
Number of lone pair electrons in nitrogen is
Number of bond pair electrons in nitrogen is
To calculate the formal charge on nitrogen, substitute the value of valence electrons, lone pair electrons and bond pair electrons in the equation (1).
Number of valence electrons in oxygen is
Number of lone pair electrons in oxygen is
Number of bond pair electrons in oxygen is
To calculate the formal charge on oxygen, substitute the value of valence electrons, lone pair electrons and bond pair electrons in the equation (1).
Number of valence electrons in carbon is
Number of lone pair electrons in carbon is
Number of bond pair electrons in carbon is
To calculate the formal charge on carbon, substitute the value of valence electrons, lone pair electrons and bond pair electrons in the equation (1).
The formal charge on each atom of resonating structure (b) of
Number of valence electrons in nitrogen is
Number of lone pair electrons in nitrogen is
Number of bond pair electrons in nitrogen is
To calculate the formal charge on nitrogen, substitute the value of valence electrons, lone pair electrons and bond pair electrons in the equation (1).
Number of valence electrons in oxygen is
Number of lone pair electrons in oxygen is
Number of bond pair electrons in oxygen is
To calculate the formal charge on oxygen, substitute the value of valence electrons, lone pair electrons and bond pair electrons in the equation (1).
Number of valence electrons in carbon is
Number of lone pair electrons in carbon is
Number of bond pair electrons in carbon is
To calculate the formal charge on carbon, substitute the value of valence electrons, lone pair electrons and bond pair electrons in the equation (1).
The formal charge on each atom of resonating structure (c) of
Number of valence electrons in nitrogen is
Number of lone pair electrons in nitrogen is
Number of bond pair electrons in nitrogen is
To calculate the formal charge on nitrogen, substitute the value of valence electrons, lone pair electrons and bond pair electrons in the equation (1).
Number of valence electrons in oxygen is
Number of lone pair electrons in oxygen is
Number of bond pair electrons in oxygen is
To calculate the formal charge on oxygen, substitute the value of valence electrons, lone pair electrons and bond pair electrons in the equation (1).
Number of valence electrons in carbon is
Number of lone pair electrons in carbon is
Number of bond pair electrons in carbon is
To calculate the formal charge on carbon, substitute the value of valence electrons, lone pair electrons and bond pair electrons in the equation (1).
According to formal charge calculations, resonating structure (a) and (c) least electronegative atom carbon possess
Conclusion
The preferred resonance form in the
The preferred resonance form in the
The preferred resonance form in the
Want to see more full solutions like this?
Chapter 8 Solutions
Chemistry
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





