
Concept explainers
(a)
To determine: The lewis structure for
(a)
Answer to Problem 8.88QP
Solution
The lewis structure for
Explanation of Solution
Explanation
Number of valence electrons in hydrogen is
Carbon is bonded to three hydrogen atoms and nitrogen atom by single bond. With one oxygen, nitrogen is bonded by single bond while with other oxygen, it is bonded by double bond. Lone pairs of electrons present on oxygen atoms are delocalized which results in the formation of another lewis structure. Hence the lewis structure of
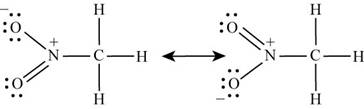
Figure 1
(b)
To determine: The lewis structure for.
(b)
Answer to Problem 8.88QP
Solution
The lewis structures for.
Explanation of Solution
Explanation
The two given possible skeletal for
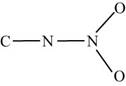
Figure 2
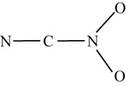
Figure 3
In the first skeletal, the nitrogen of
Number of valence electrons in carbon is
With one oxygen atom, nitrogen is bonded by single bond while with other oxygen; it is bonded by double bond. Lone pairs of electrons present on oxygen atoms are delocalized which results in the formation of another lewis structure. Hence the lewis structure of
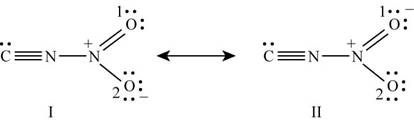
Figure 4
The formal charge on each atom of resonating structure (I) of
Formal charge is calculated as,
Number of valence electrons in first nitrogen is
Number of lone pair electrons in first nitrogen is
Number of bond pair electrons in first nitrogen is
To calculate the formal charge on first nitrogen, substitute the value of valence electrons, lone pair electrons and bond pair electrons in the equation (1).
Number of valence electrons in carbon is
Number of lone pair electrons in carbon is
Number of bond pair electrons in carbon is
To calculate the formal charge on carbon, substitute the value of valence electrons, lone pair electrons and bond pair electrons in the equation (1).
Number of valence electrons in second nitrogen is
Number of lone pair electrons in second nitrogen is
Number of bond pair electrons in second nitrogen is
To calculate the formal charge on second nitrogen, substitute the value of valence electrons, lone pair electrons and bond pair electrons in the equation (1).
Number of valence electrons in oxygen (1) is
Number of lone pair electrons in oxygen (1) is
Number of bond pair electrons in oxygen (1) is
To calculate the formal charge on oxygen (1), substitute the value of valence electrons, lone pair electrons and bond pair electrons in the equation (1).
Number of valence electrons in oxygen (2) is
Number of lone pair electrons in oxygen (2) is
Number of bond pair electrons in oxygen (2) is
To calculate the formal charge on oxygen (2), substitute the value of valence electrons, lone pair electrons and bond pair electrons in the equation (1).
The formal charge on each atom of resonating structure (II) of
Number of valence electrons in first nitrogen is
Number of lone pair electrons in first nitrogen is
Number of bond pair electrons in first nitrogen is
To calculate the formal charge on first nitrogen, substitute the value of valence electrons, lone pair electrons and bond pair electrons in the equation (1).
Number of valence electrons in carbon is
Number of lone pair electrons in carbon is
Number of bond pair electrons in carbon is
To calculate the formal charge on carbon, substitute the value of valence electrons, lone pair electrons and bond pair electrons in the equation (1).
Number of valence electrons in second nitrogen is
Number of lone pair electrons in second nitrogen is
Number of bond pair electrons in second nitrogen is
To calculate the formal charge on second nitrogen, substitute the value of valence electrons, lone pair electrons and bond pair electrons in the equation (1).
Number of valence electrons in oxygen (1) is
Number of lone pair electrons in oxygen (1) is
Number of bond pair electrons in oxygen (1) is
To calculate the formal charge on oxygen (1), substitute the value of valence electrons, lone pair electrons and bond pair electrons in the equation (1).
Number of valence electrons in oxygen (2) is
Number of lone pair electrons in oxygen (2) is
Number of bond pair electrons in oxygen (2) is
To calculate the formal charge on oxygen (2), substitute the value of valence electrons, lone pair electrons and bond pair electrons in the equation (1).
Number of valence electrons in carbon is
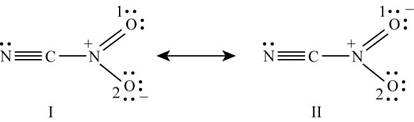
Figure 5
The formal charge on each atom of resonating structure (I) of
Number of valence electrons in first nitrogen is
Number of lone pair electrons in first nitrogen is
Number of bond pair electrons in first nitrogen is
To calculate the formal charge on first nitrogen, substitute the value of valence electrons, lone pair electrons and bond pair electrons in the equation (1).
Number of valence electrons in carbon is
Number of lone pair electrons in carbon is
Number of bond pair electrons in carbon is
To calculate the formal charge on carbon, substitute the value of valence electrons, lone pair electrons and bond pair electrons in the equation (1).
Number of valence electrons in second nitrogen is
Number of lone pair electrons in second nitrogen is
Number of bond pair electrons in second nitrogen is
To calculate the formal charge on second nitrogen, substitute the value of valence electrons, lone pair electrons and bond pair electrons in the equation (1).
Number of valence electrons in oxygen (1) is
Number of lone pair electrons in oxygen (1) is
Number of bond pair electrons in oxygen (1) is
To calculate the formal charge on oxygen (1), substitute the value of valence electrons, lone pair electrons and bond pair electrons in the equation (1).
Number of valence electrons in oxygen (2) is
Number of lone pair electrons in oxygen (2) is
Number of bond pair electrons in oxygen (2) is
To calculate the formal charge on oxygen (2), substitute the value of valence electrons, lone pair electrons and bond pair electrons in the equation (1).
The formal charge on each atom of resonating structure (II) of
Number of valence electrons in first nitrogen is
Number of lone pair electrons in first nitrogen is
Number of bond pair electrons in first nitrogen is
To calculate the formal charge on first nitrogen, substitute the value of valence electrons, lone pair electrons and bond pair electrons in the equation (1).
Number of valence electrons in carbon is
Number of lone pair electrons in carbon is
Number of bond pair electrons in carbon is
To calculate the formal charge on carbon, substitute the value of valence electrons, lone pair electrons and bond pair electrons in the equation (1).
Number of valence electrons in second nitrogen is
Number of lone pair electrons in second nitrogen is
Number of bond pair electrons in second nitrogen is
To calculate the formal charge on second nitrogen, substitute the value of valence electrons, lone pair electrons and bond pair electrons in the equation (1).
Number of valence electrons in oxygen (1) is
Number of lone pair electrons in oxygen (1) is
Number of bond pair electrons in oxygen (1) is
To calculate the formal charge on oxygen (1), substitute the value of valence electrons, lone pair electrons and bond pair electrons in the equation (1).
Number of valence electrons in oxygen (2) is
Number of lone pair electrons in oxygen (2) is
Number of bond pair electrons in oxygen (2) is
To calculate the formal charge on oxygen (2), substitute the value of valence electrons, lone pair electrons and bond pair electrons in the equation (1).
The resonating structure which possesses zero or minimum formal charge is preferred. The distribution of formal charges on both the molecules
(c)
To determine: Whether the given two structures of
(c)
Answer to Problem 8.88QP
Solution
The two structures of
Explanation of Solution
Explanation
In the resonating forms, the postion of atoms remains same. But in the two structures of
Conclusion
The two structures of
Want to see more full solutions like this?
Chapter 8 Solutions
Chemistry
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





